
Exhibit 99.1

CollPlant Overview HCW Global Investment Conference September 2019

Safe Harbor Statement Certain statements in this presentation constitute "forward - looking statements" within the meaning of Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act and are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or similar expressions . We intend these forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions . These forward - looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made . Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved . Furthermore, actual results may differ materially from those described in the forward - looking statements and will be affected by a variety of risks and factors that are beyond our control . Risks and uncertainties for our company include, but are not limited to : the Company’s history of significant losses, its ability to continue as a going concern, and its need to raise additional capital and its inability to obtain additional capital on acceptable terms, or at all ; the Company’s expectations regarding the timing and cost of commencing clinical trials with respect to tissues and organs which are based on its rhCollagen based Bioink, dermal fillers for aesthetics, breast implants, VergenixSTR, and VergenixFG ; the Company’s ability to obtain favorable pre - clinical and clinical trial results ; regulatory action with respect to rhCollagen based BioInk, dermal fillers for aesthetics, breast implants, VergenixSTR, and VergenixFG including but not limited to acceptance of an application for marketing authorization, review and approval of such application, and, if approved, the scope of the approved indication and labeling ; commercial success and market acceptance of the Company’s rhCollagen based BioInk, dermal fillers for aesthetics, VergenixSTR, and VergenixFG ; the Company’s ability to establish sales and marketing capabilities or enter into agreements with third parties and its reliance on third party distributors and resellers ; the Company’s ability to establish and maintain strategic partnerships and other corporate collaborations ; the Company’s reliance on third parties to conduct some or all aspects of its product manufacturing ; the scope of protection the Company is able to establish and maintain for intellectual property rights and the Company’s ability to operate its business without infringing the intellectual property rights of others ; the overall global economic environment ; the impact of competition and new technologies ; general market, political, and economic conditions in the countries in which the Company operates ; projected capital expenditures and liquidity ; changes in the Company’s strategy ; and litigation and regulatory proceedings . Many of these factors that will determine actual results are beyond our ability to control or predict . For a discussion of the factors that may cause our actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied in such forward - looking statements, see the “Risk Factors” section of included in our most recently filed Annual Report on Form 20 - F . Existing and prospective investors are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof . The statements made in this presentation speak only as of the date stated herein, and subsequent events and developments may cause our expectations and beliefs to change . Unless otherwise required by applicable securities laws, we do not intend, nor do we undertake any obligation, to update or revise any forward - looking statements contained in this presentation to reflect subsequent information, events, results or circumstances or otherwise . While we may elect to update these forward - looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise, except as required by law . The trademarks included herein are the property of the owners thereof and are used for reference purposes only . Such use should not be construed as an endorsement of such products . 2
O v e r view ● Deep Tech * company developing, manufacturing and commercializing regenerative medicine products ● Proprietary technology platform that enables mass production of recombinant human collagen (rhCollagen) ● rhCollagen - based products aimed at 3D Bioprinting of tissues and organs, and medical aesthetics markets ● Strategic agreement with United Therapeutics (NASDAQ: UTHR) for 3D bioprinting of lung transplants and other life saving organs 3 * Deep Technologies are novel technologies that offer significant advances over those currently in use. (Source: The Dawn of the Deep Tech Ecosystem, BCG, March 2019)

Prof. Oded Shoseyov Co - Founder & Chief Scientist Pauli Clean Tech CBD Tech. F u l cru m - SPD Melodea Hebrew University Eran Rotem Deputy CEO & CFO Tefron, CFO (NYSE,TASE) Healthcare Tech., CFO (NASDAQ) & Gamida E&Y Ilana Belzer, PhD COO BioHarvest Procognia Ltd. Omrix Biopharmaceuticals Interpharm Nadav Orr, PhD VP R&D Ethicon Biosurgery, Johnson & Johnson 4 Philippe Bensimon, PharmD VP RA/QA/CA Maquet Getinge 3M Medical Yehiel Tal CEO Regentis Biomaterials ProChon Biotech Kulicke & Soffa Industries Experienced management team
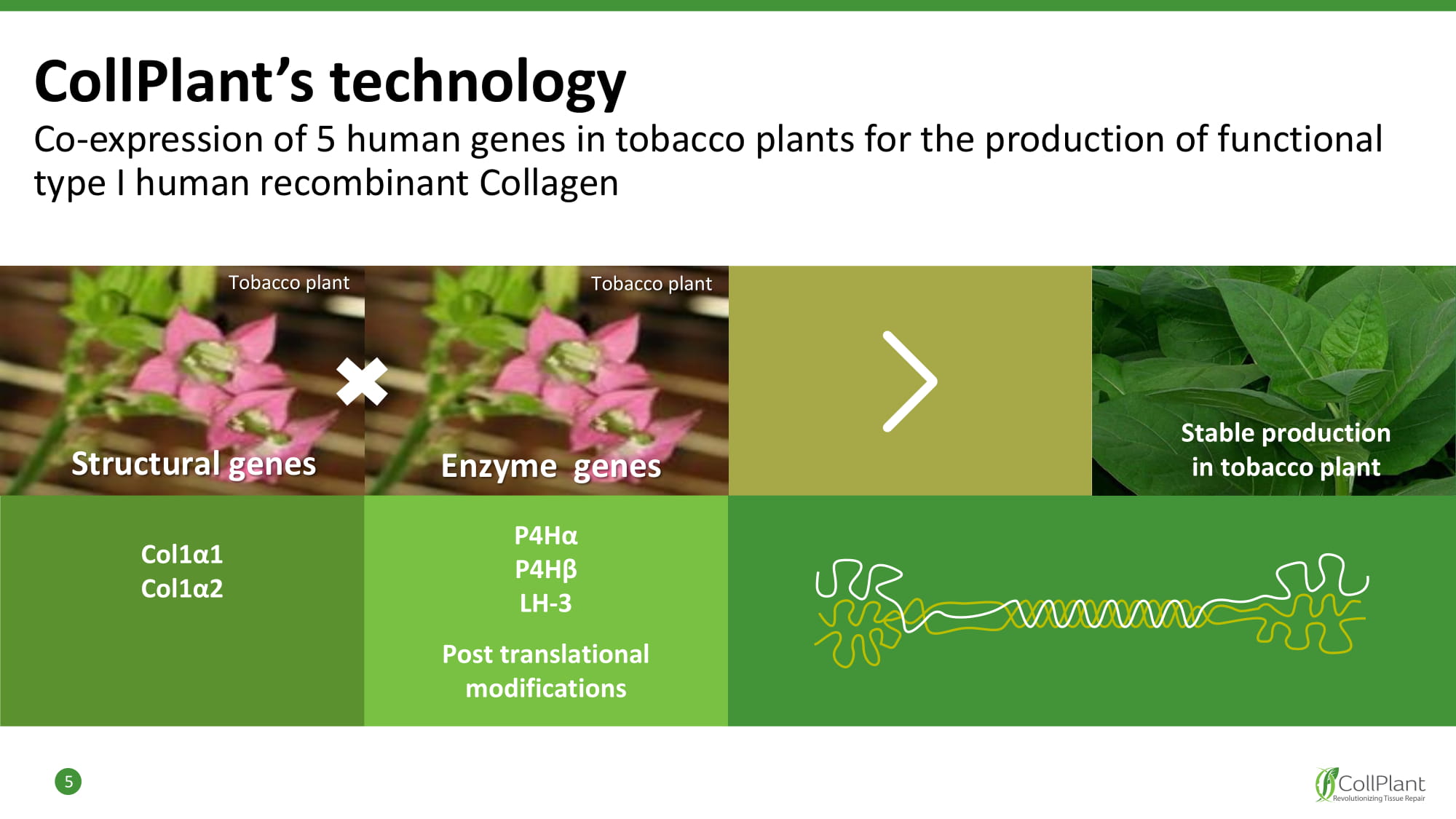
CollPlant’s technology Co - expression of 5 human genes in tobacco plants for the production of functional type I human recombinant Collagen 5 P 4 Hα P 4 Hβ LH - 3 Post translational modifications Co l 1α 1 Co l 1α 2 Stable production in tobacco plant Structural genes Enzyme genes Tobacco plant Tobacco plant
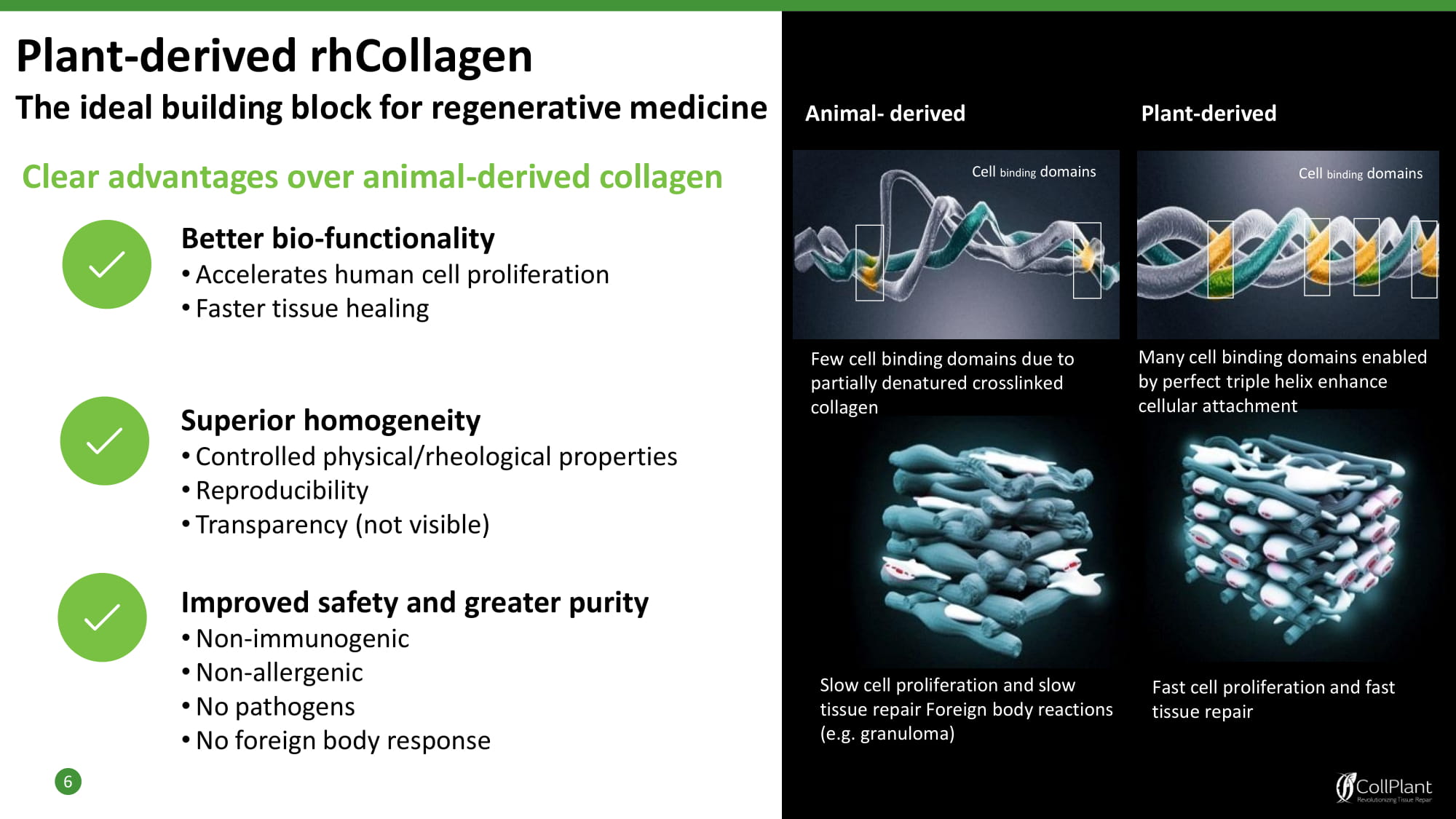
Plant - derived rhCollagen The ideal building block for regenerative medicine 6 Animal - derived Cell binding domains Cell binding domains Few cell binding domains due to partially denatured crosslinked collagen Many cell binding domains enabled by perfect triple helix enhance cellular attachment Slow cell proliferation and slow tissue repair Foreign body reactions (e.g. granuloma) Fast cell proliferation and fast tissue repair Plant - derived Superior homogeneity • Controlled physical/rheological properties • Reproducibility • Transparency (not visible) Improved safety and greater purity • Non - immunogenic • Non - allergenic • No pathogens • No foreign body response Clear advantages over animal - derived collagen Better bio - functionality • Accelerates human cell proliferation • Faster tissue healing
Medical ae s th e tics R e g ene r a t i v e dermal filler
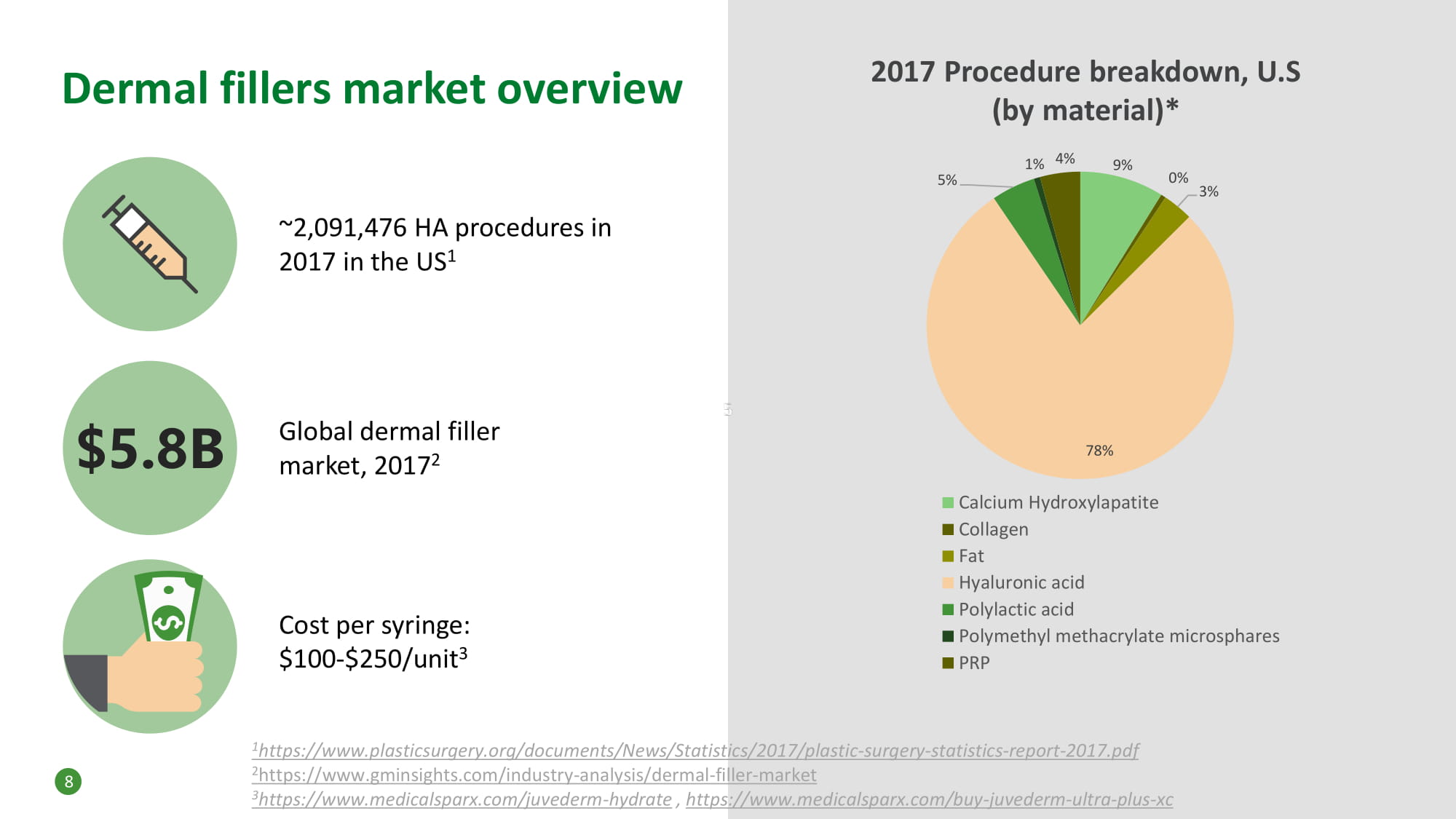
Dermal fillers market overview ~2,091,476 HA procedures in 2017 in the US 1 Global dermal filler market, 2017 2 Cost per syringe: $100 - $250/unit 3 9% 0% 3% 78% 5% 1% 4% Calcium Hydroxylapatite Collagen Fat Hyaluronic acid Polylactic acid Polymethyl methacrylate microsphares PRP 2017 Procedure breakdown, U.S (by material)* $5.8B 1 https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic - surgery - statistics - report - 2017.pdf 2 https://www.gminsights.com/industry - analysis/dermal - filler - market 3 https://www.medicalsparx.com/juvederm - hydrate , https://www.medicalsparx.com/buy - juvederm - ultra - plus - xc 8
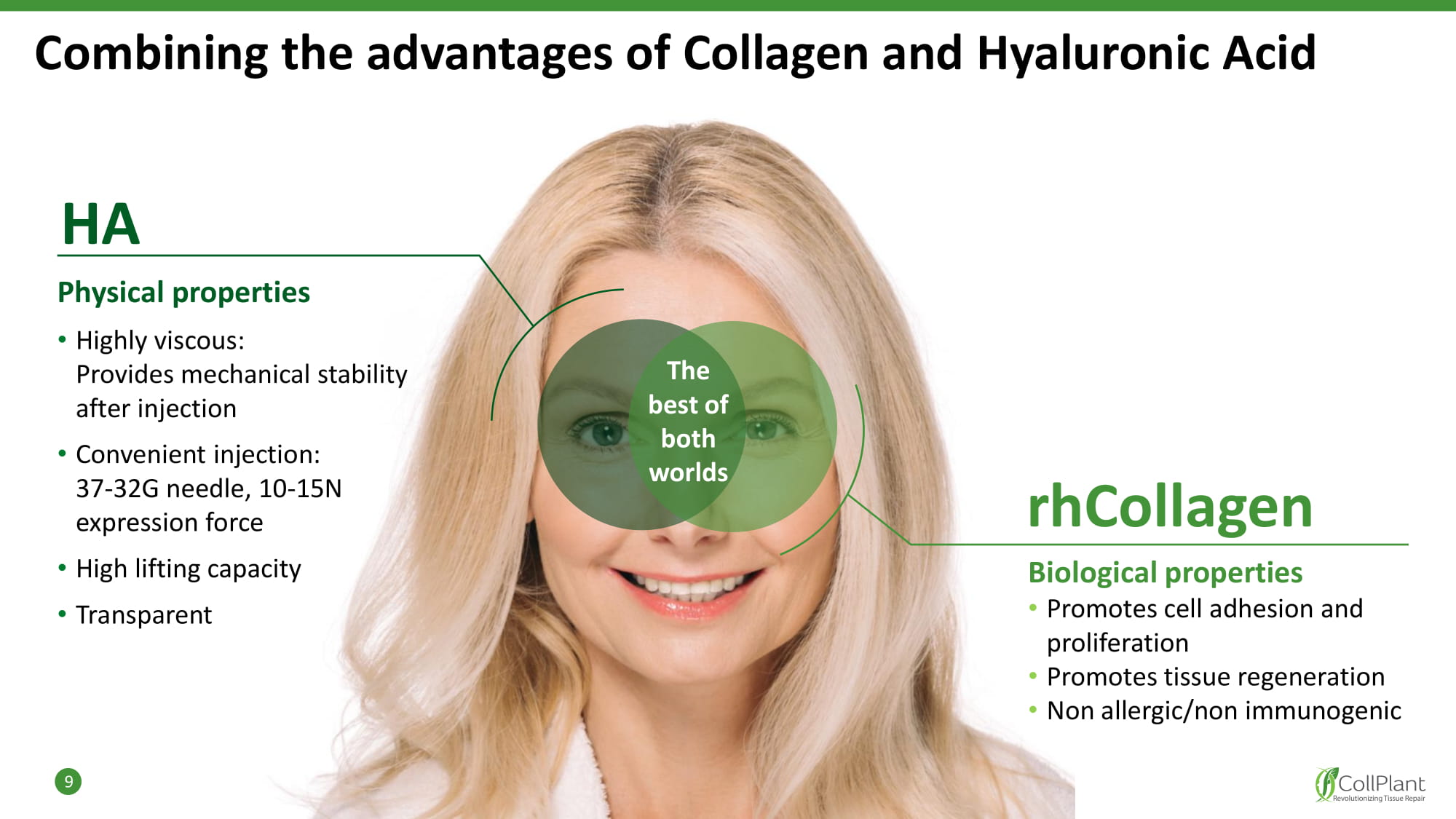
HA Physical properties • Highly viscous: Provides mechanical stability after injection • Convenient injection: 37 - 32G needle, 10 - 15N expression force • High lifting capacity • Transparent Combining the advantages of Collagen and Hyaluronic Acid 9 rhCollagen Biological properties • Promotes cell adhesion and proliferation • Promotes tissue regeneration • Non allergic/non immunogenic The best of both w orlds
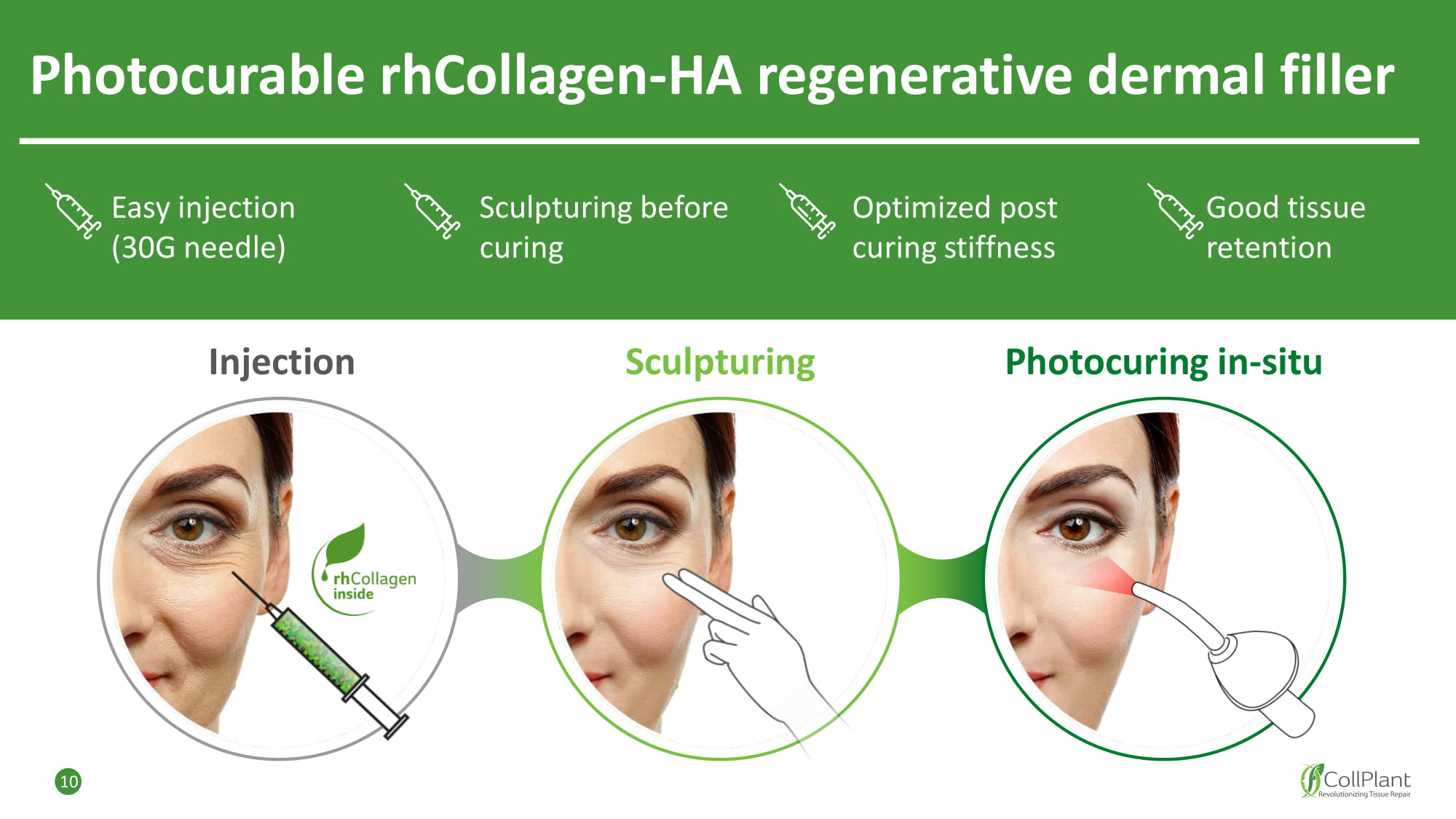
Photocurable rhCollagen - HA regenerative dermal filler Good tissue retention Sculpturing before curing Easy injection (30G needle) I n je c tion Scul p turing Photocuring in - situ Optimized post curing stiffness 10

3D Bioprinting tissues & organs
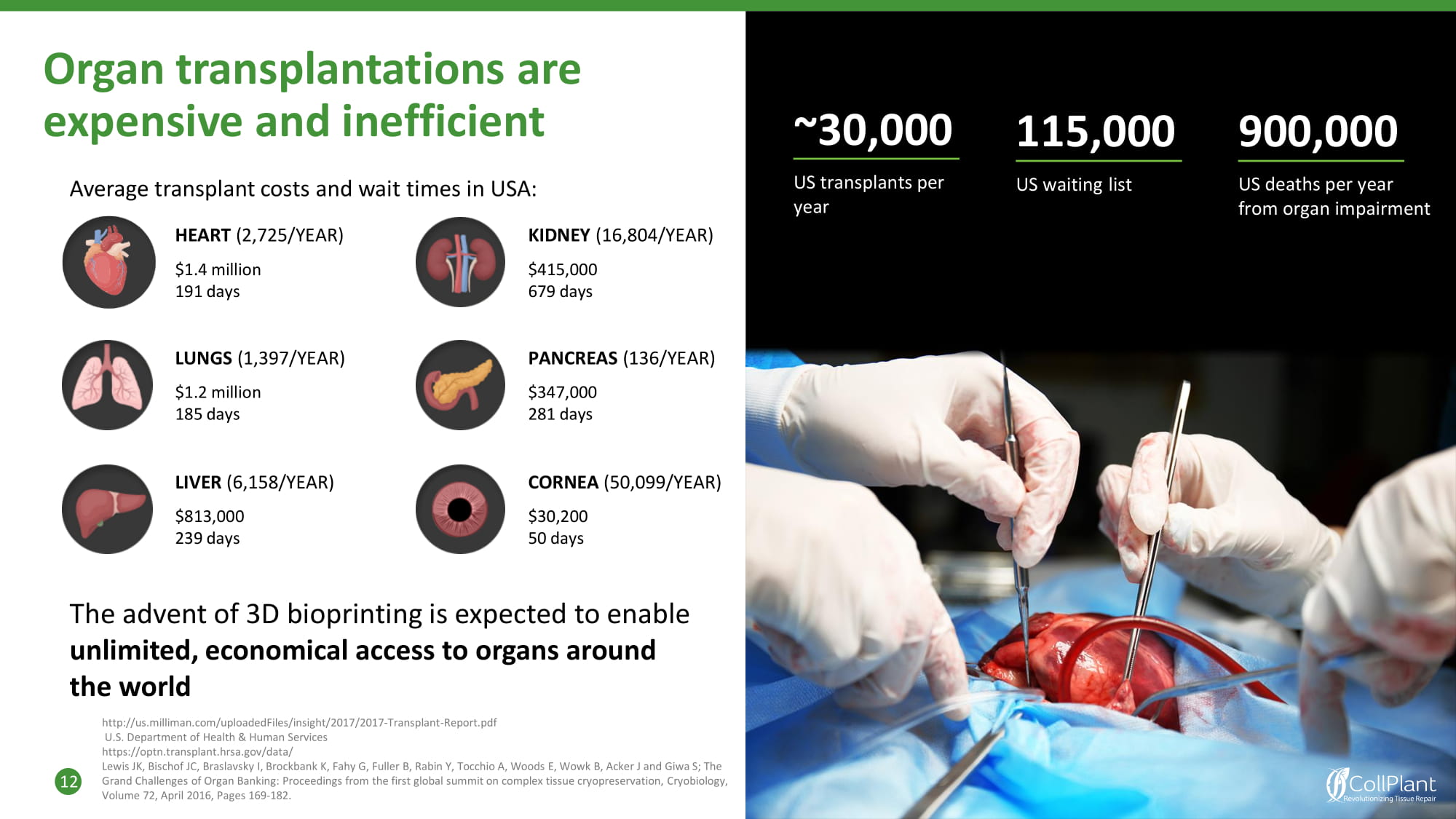
Organ transplantations are expensive and inefficient Grand Challenges of Organ Banking: Proceedings from the first global summit on complex tissue cryopreservation, Cryobiology, Volume 72, April 2016, Pages 169 - 182. 12 Average transplant costs and wait times in USA: The advent of 3D bioprinting is expected to enable unlimited, economical access to organs around the world http://us.milliman.com/uploadedFiles/insight/2017/2017 - Transplant - Report.pdf U.S. Department of Health & Human Services https://optn.transplant.hrsa.gov/data/ Lewis JK, Bischof JC, Braslavsky I, Brockbank K, Fahy G, Fuller B, Rabin Y, Tocchio A, Woods E, Wowk B, Acker J and Giwa S; The KIDNEY (16,804/YEAR) $415,000 679 days ~3 0 ,000 US transplants per year 1 1 5,0 0 0 US waiting list 900,000 US deaths per year from organ impairment HEART (2,725/YEAR) $1.4 million 191 days LUNGS (1,397/YEAR) $1.2 million 185 days PANCREAS (136/YEAR) $347,000 281 days LIVER (6,158/YEAR) $813,000 239 days CORNEA (50,099/YEAR) $30,200 50 days
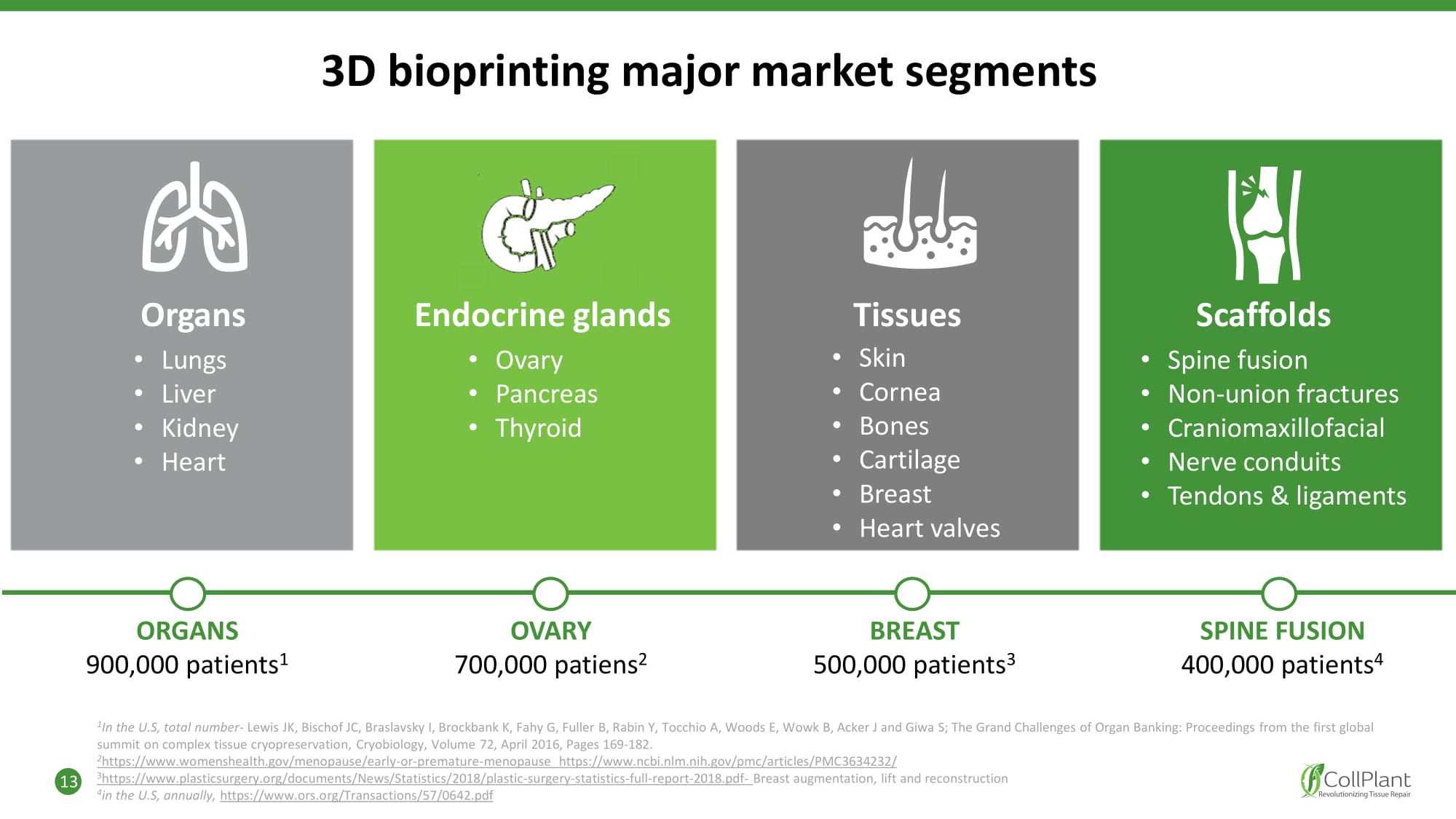
3D bioprinting major market segments • Lungs • Liver • Kid n e y • Heart O r g ans • Ovary • P a nc r eas • Thyroid Scaffolds • Spine fusion • Non - union fractures • Craniomaxillofacial • Nerve conduits • Tendons & ligaments Endocrine glands 1 In the U.S, total number - Lewis JK, Bischof JC, Braslavsky I, Brockbank K, Fahy G, Fuller B, Rabin Y, Tocchio A, Woods E, Wowk B, Acker J and Giwa S; The Grand Challenges of Organ Banking: Proceedings from the first global summit on complex tissue cryopreservation, Cryobiology, Volume 72, April 2016, Pages 169 - 182. 2 https://www.womenshealth.gov/menopause/early - or - premature - menopause https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3634232/ 3 https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic - surgery - statistics - full - report - 2018.pdf - Breast augmentation, lift and reconstruction 4 in the U.S, annually, https://www.ors.org/Transactions/57/0642.pdf OVARY 700,000 patiens 2 BREAST 500,000 patients 3 SPINE FUSION 400,000 patients 4 ORGANS 900,000 patients 1 Tissues • Skin • Cornea • Bones • Cartilage • Breast • Heart valves 13
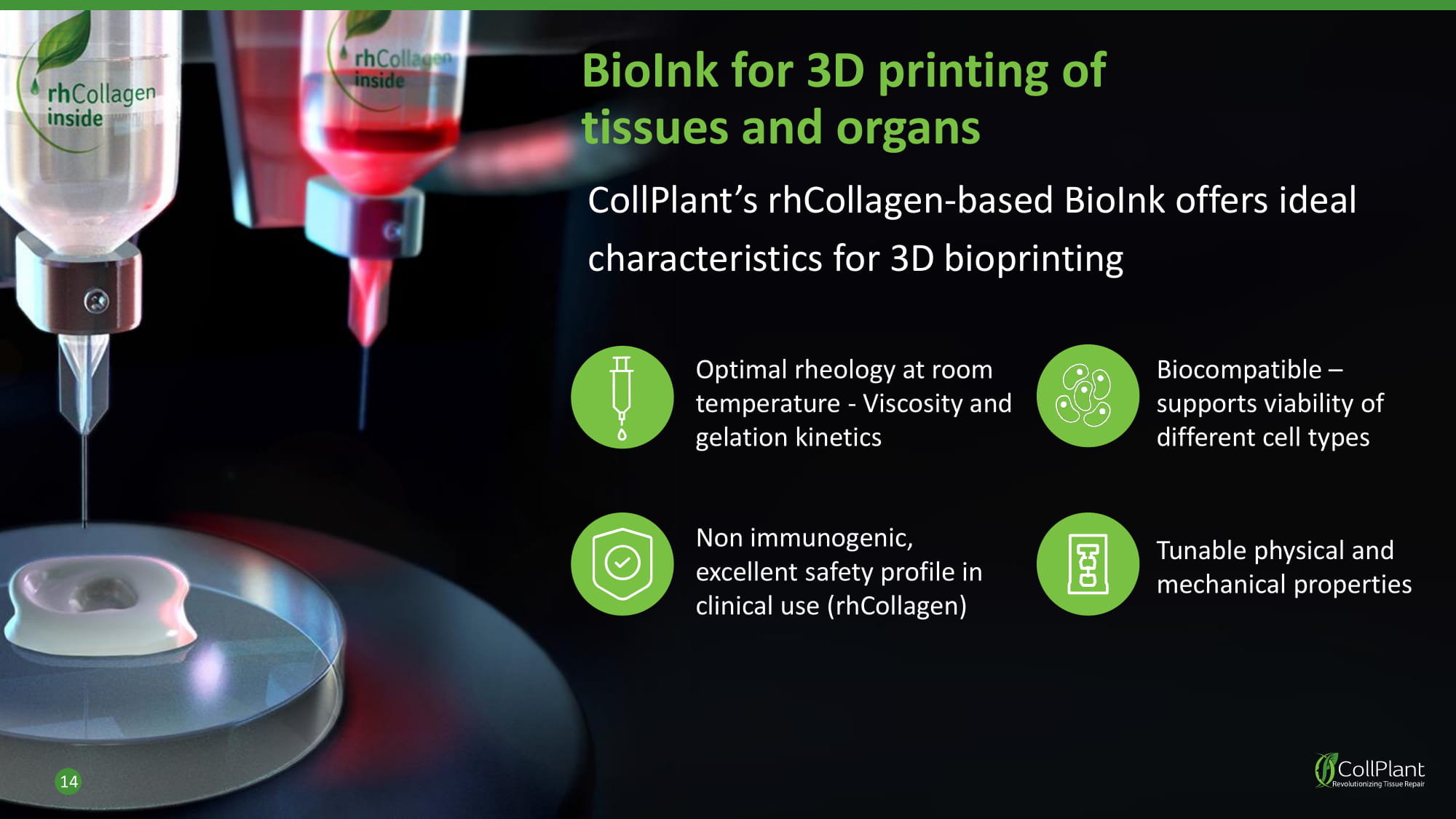
BioInk for 3D printing of tissues and organs CollPlant’s rhCollagen - based BioInk offers ideal characteristics for 3D bioprinting 14 Optimal rheology at room temperature - Viscosity and gelation kinetics Non immunogenic, excellent safety profile in clinical use (rhCollagen) Biocompatible – supports viability of different cell types Tunable physical and mechanical properties
15 Collaboration agreement with United Therapeutics (Oct. 2018) • Global licensing and commercialization agreement for 3D Bioprinting of solid organ scaffolds for human transplants • Collaboration combines CollPlant’s proprietary BioInk technology and United Therapeutics’ regenerative medicine and organ manufacturing capabilities Agreement highlights: • $5M upfront payment • Up to $39M milestone and option payments • Royalties on product sales • United Therapeutics has the option to expand the license to add up to three organs • United Therapeutics will establish a U.S. facility for the manufacture of CollPlant's rhCollagen and BioInk

16 3D bioprinting of trachea and bronchi Courtesy of United Therapeutics 100µ perfused vessels

Breast impla n ts
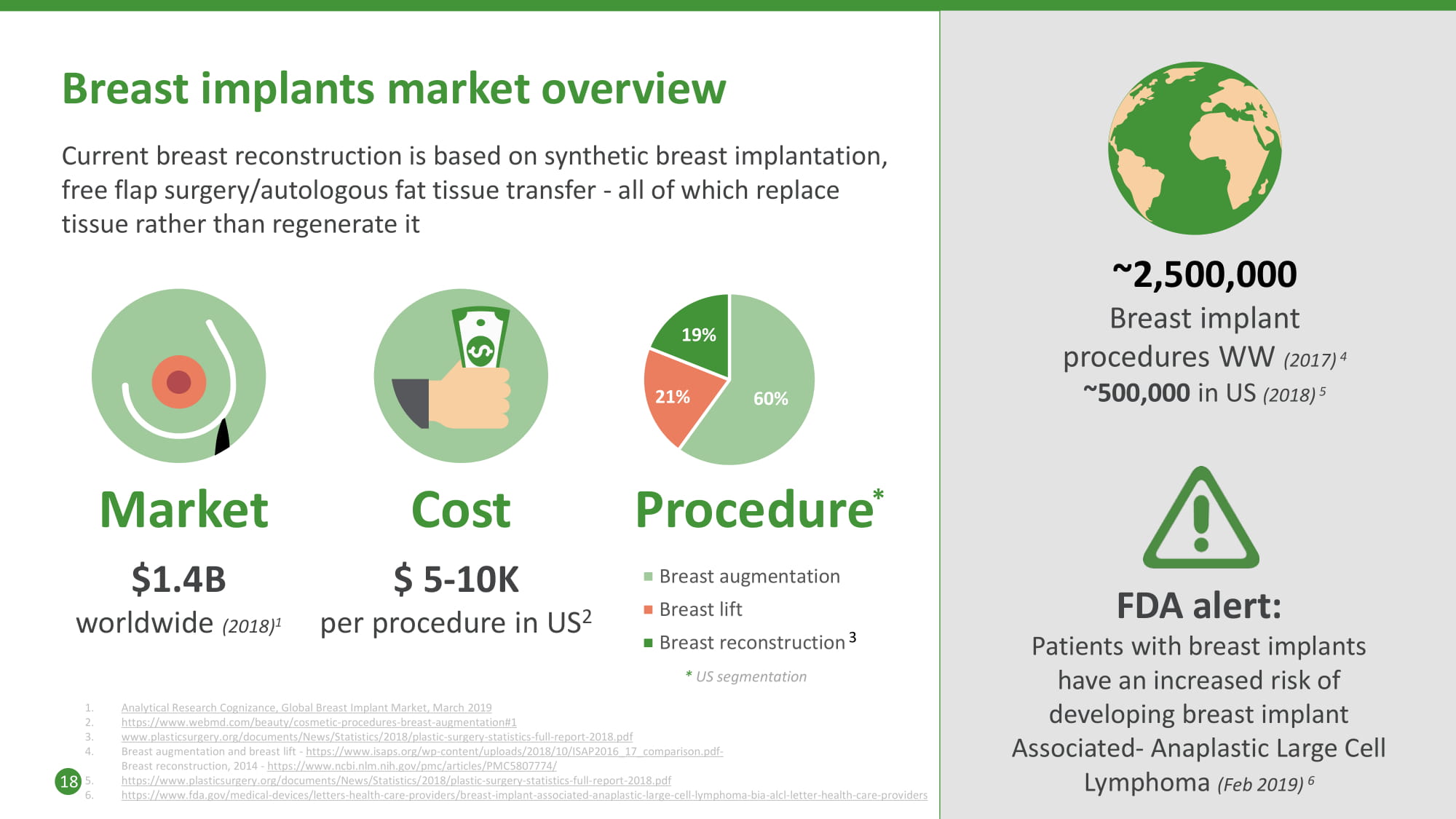
60 % 21 % 19 % Breast implants market overview Cost $ 5 - 10K per procedure in US 2 ~2,500,000 Breast implant procedures WW (2017) 4 ~500,000 in US (2018) 5 Market $1.4B worldwide (2018) 1 Current breast reconstruction is based on synthetic breast implantation, free flap surgery/autologous fat tissue transfer - all of which replace tissue rather than regenerate it 1. 2. 3. 4. Analytical Research Cognizance, Global Breast Implant Market, March 2019 https://www.webmd.com/beauty/cosmetic - procedures - breast - augmentation#1 www.plasticsurgery.org/documents/News/Statistics/2018/plastic - surgery - statistics - full - report - 2018.pd f Breast augmentation and breast lift - https://www.isaps.org/wp - content/uploads/2018/10/ISAP2016_17_comparison.pdf - Breast reconstruction, 2014 - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5807774/ https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic - surgery - statistics - full - report - 2018.pd f https://www.fda.gov/medical - devices/letters - health - care - providers/breast - implant - associated - anaplastic - large - cell - lymphoma - bia - a lcl - letter - health - care - providers FDA alert: Patients with breast implants have an increased risk of developing breast implant Associated - Anaplastic Large Cell Lymphoma (Feb 2019) 6 18 5. 6. Procedure * Breast augmentation Breast lift Breast reconstruction 3 * US segmentation
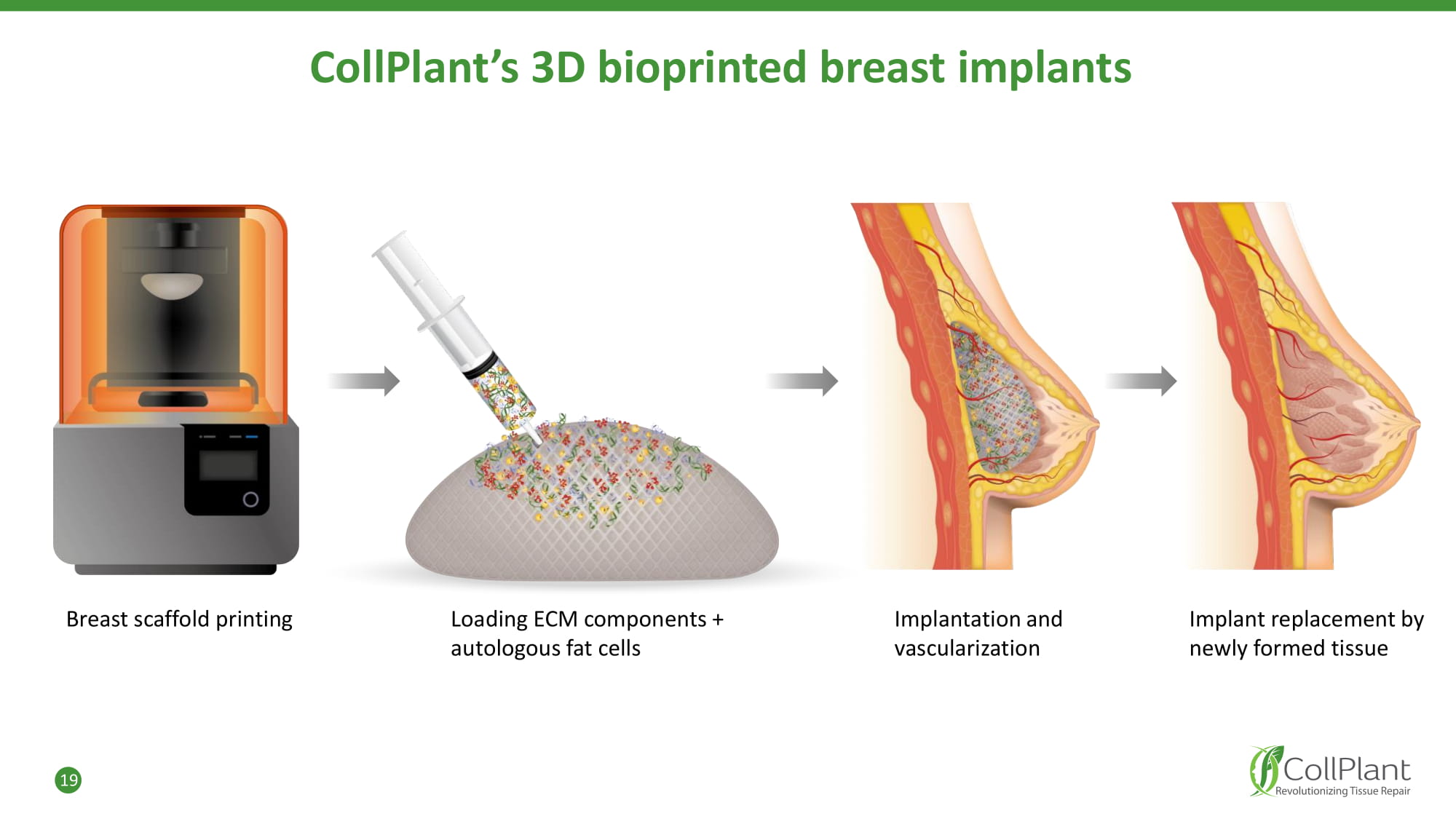
19 CollPlant’s 3D bioprinted breast implants Breast scaffold printing Loading ECM components + autologous fat cells Implantation and vascularization Implant replacement by newly formed tissue

20 NASDAQ (CLGN) listed ADR since Jan. 2018 Market Cap of ~ $16M * 45 employees • Strong R&D team • Fully integrated • Production team with eight years track record * As of August 30, 2019
Planned 12 - month milestones ● Medical aesthetics - Sign collaboration agreement with strategic partner - Photocurable dermal filler animal study ● 3D Bioprinting - Expand collaborations with key players - Breast implants animal study 21

CollPlant investment highlights Only commercially viable technology currently available that can produce truly human collagen Multi - billion dollar market: innovative rhCollagen products initially aimed at 3D bioprinting and medical aesthetics Broadly applicable technology: Ideal building block/scaffolding molecule for regenerative medicine Strategic agreement with United Therapeutics (UTHR) for 3D bioprinting of lungs and other life saving organs Clinically validated technology Proven management team 22

Thank you