
Exhibit 99.1

CollPlant Overview April 2022

Safe harbor statement Certain statements in this presentation constitute "forward - looking statements" within the meaning of Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act and are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or similar expressions . We intend these forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions . These forward - looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made . Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved . Furthermore, actual results may differ materially from those described in the forward - looking statements and are expected to be affected by a variety of risks and factors that are beyond our control . Risks and uncertainties for our company include, but are not limited to : the Company’s history of significant losses and its need to raise additional capital and its inability to obtain additional capital on acceptable terms, or at all ; the Company’s expectations regarding the timing and cost of commencing clinical trials with respect to tissues and organs which are based on its rhCollagen based Bioink and products for medical aesthetics ; the Company’s ability to obtain favorable pre - clinical and clinical trial results ; regulatory action with respect to rhCollagen based BioInkand medical aesthetics products including but not limited to acceptance of an application for marketing authorization, review and approval of such application, and, if approved, the scope of the approved indication and labeling ; commercial success and market acceptance of the Company’s rhCollagen based products, in 3 D bioprinting and medical aesthetics ; the Company’s ability to establish sales and marketing capabilities or enter into agreements with third parties and its reliance on third party distributors and resellers ; the Company’s ability to establish and maintain strategic partnerships and other corporate collaborations ; the Company’s reliance on third parties to conduct some or all aspects of its product manufacturing ; the scope of protection the Company is able to establish and maintain for intellectual property rights and the Company’s ability to operate its business without infringing the intellectual property rights of others ; the overall global economic environment ; the impact of competition and new technologies ; general market, political, and economic conditions in the countries in which the Company operates ; projected capital expenditures and liquidity ; changes in the Company’s strategy ; and litigation and regulatory proceedings . Many of these factors that will determine actual results are beyond our ability to control or predict . For a discussion of the factors that may cause our actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied in such forward - looking statements, see the “Risk Factors” section of included in our most recently filed Annual Report on Form 20 - F . Existing and prospective investors are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof . The statements made in this presentation speak only as of the date stated herein, and subsequent events and developments may cause our expectations and beliefs to change . Unless otherwise required by applicable securities laws, we do not intend, nor do we undertake any obligation, to update or revise any forward - looking statements contained in this presentation to reflect subsequent information, events, results or circumstances or otherwise . While we may elect to update these forward - looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise, except as required by law . The trademarks included herein are the property of the owners thereof and are used for reference purposes only . Such use should not be construed as an endorsement of such products .

3 CollPlant – Unlocking the Regenerative Potential of rhCollagen Collaboration Agreements with Industry Leaders • AbbVie (ABBV) - Worldwide exclusive agreement for dermal and soft tissue fillers. Up to $103mm in payments plus royalties. • 3D Systems (DDD) - Bioprinted solutions for breast reconstruction treatments Well Capitalized $ 43 mm in cash Zero debt (December 31, 2021) Regenerative and Aesthetic Medicine Company Developing innovative technologies and products for tissue regeneration and organ manufacturing, to help people live longer and better Proprietary Plant - based Technology Platform Only commercially viable solution for mass production of rhCollagen
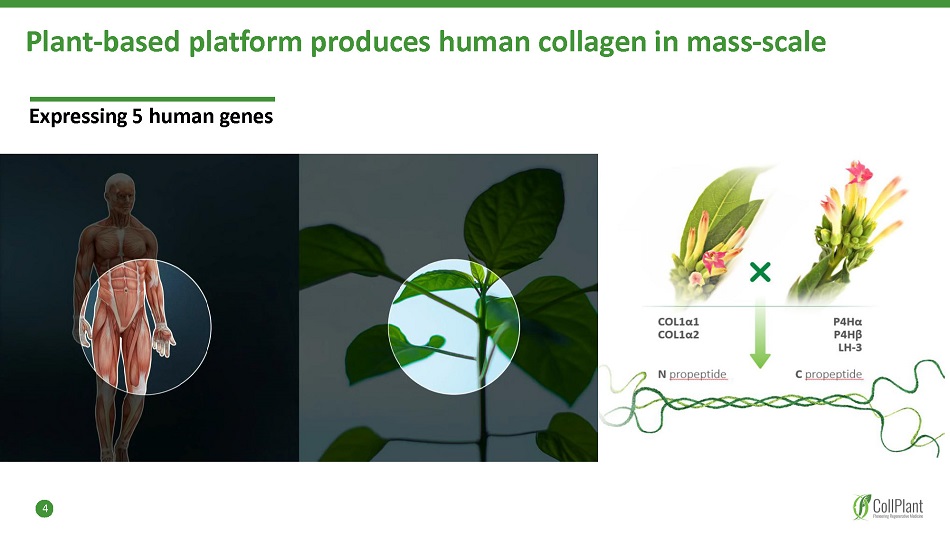
4 Expressing 5 human genes Plant - based platform produces human collagen in mass - scale
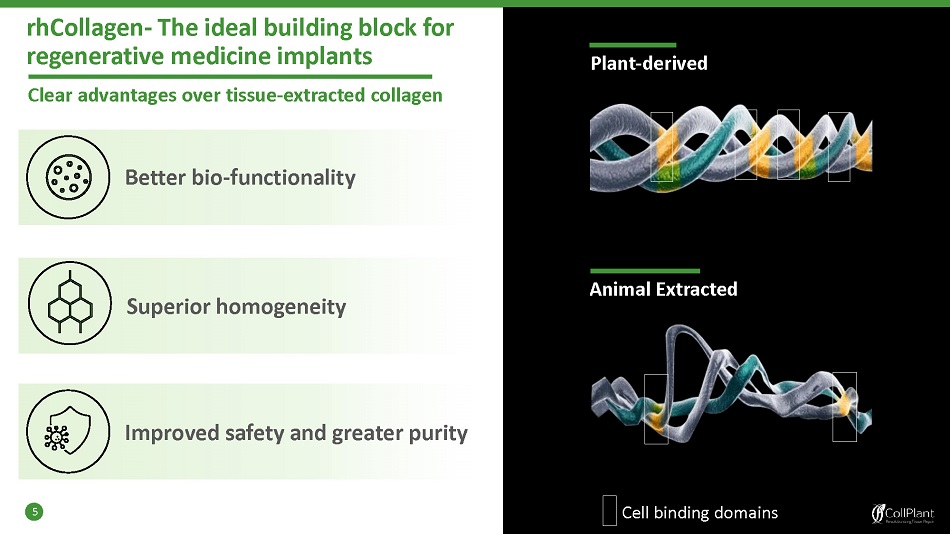
rhCollagen - The ideal building block for regenerative medicine implants 5 Animal Extracted Plant - derived Clear advantages over tissue - extracted collagen Better bio - functionality Superior homogeneity Improved safety and greater purity Cell binding domains
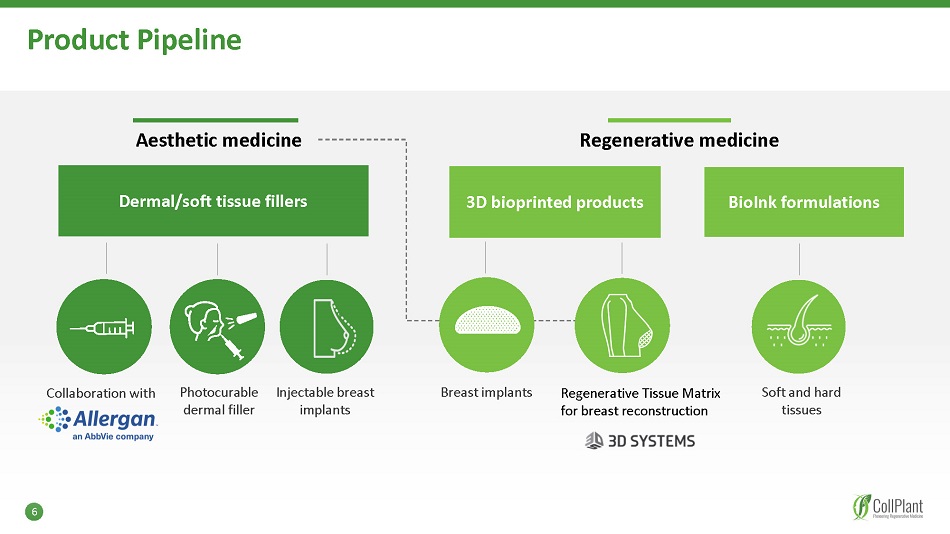
Product Pipeline Aesthetic medicine Regenerative medicine Dermal/soft tissue fillers BioInk formulations Photocurable dermal filler Injectable breast implants Soft and hard tissues Collaboration with 3D bioprinted products Breast implants Regenerative Tissue Matrix for breast reconstruction
Dermal/Soft Tissue Fillers In collaboration with

Dermal fillers market overview ~2.6 M HA procedures in 2020 in the US 1 $5B, 9.6% CAGR Global dermal filler market, 2021, CAGR 2022 - 2028 2 $100 - $250/unit Cost per syringe 3 8 1. 2. 3. https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic - surgery - statistics - full - report - 2020.pdf https:// www.gminsights.com/industry - analysis/dermal - filler - market https://www.medicalsparx.com/juvederm - hydrate , https://www.medicalsparx.com/buy - juvederm - ultra - plus - xc Hyaluronic acid 77% 2020 Procedure breakdown, U.S (by material) 1 Polymethyl methacrylate microspheres 2% Fat 4% Polylactic acid 4% PRP 7% Calcium Hydroxylapatite 6%
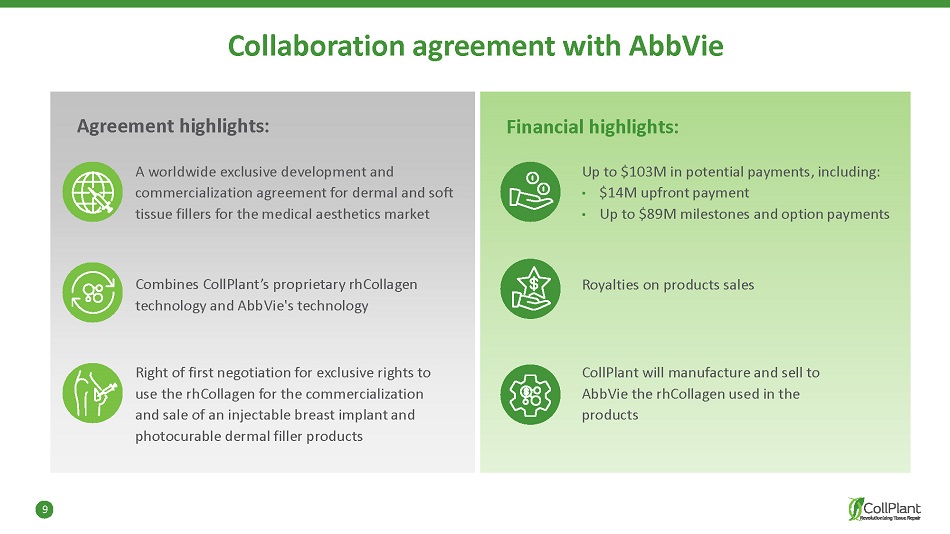
9 Collaboration agreement with AbbVie Agreement highlights: A worldwide exclusive development and commercialization agreement for dermal and soft tissue fillers for the medical aesthetics market Financial highlights: Up to $103M in potential payments, including: • $14M upfront payment • Up to $89M milestones and option payments Combines CollPlant’s proprietary rhCollagen technology and AbbVie's technology Right of first negotiation for exclusive rights to use the rhCollagen for the commercialization and sale of an injectable breast implant and photocurable dermal filler products CollPlant will manufacture and sell to AbbVie the rhCollagen used in the products Royalties on products sales
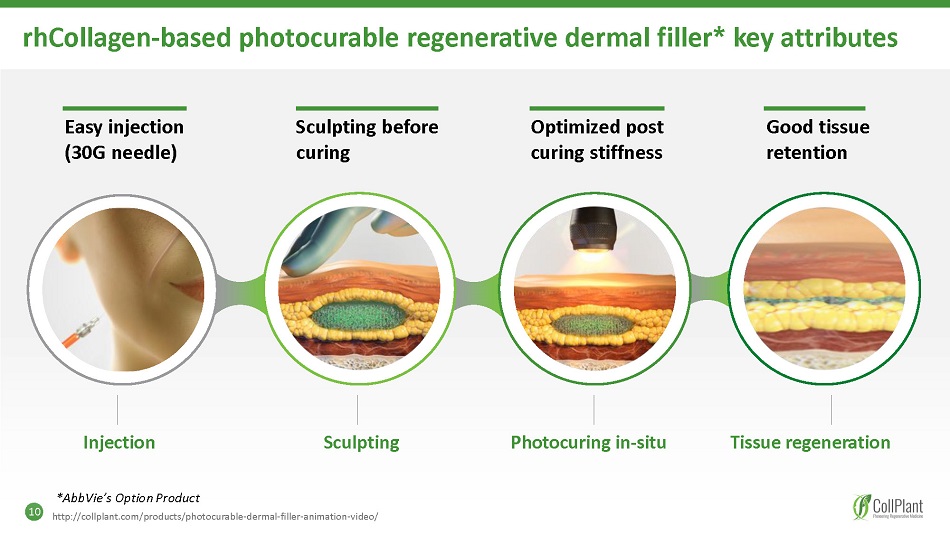
rhCollagen - based photocurable regenerative dermal filler * key attributes Photocuring in - situ Tissue regeneration Good tissue retention Sculpting before curing Easy injection (30G needle) Optimized post curing stiffness Injection Sculpting *AbbVie’s Option Product http://collplant.com/products/photocurable - dermal - filler - animation - video/

3D Bioprinted Tissues and Organs
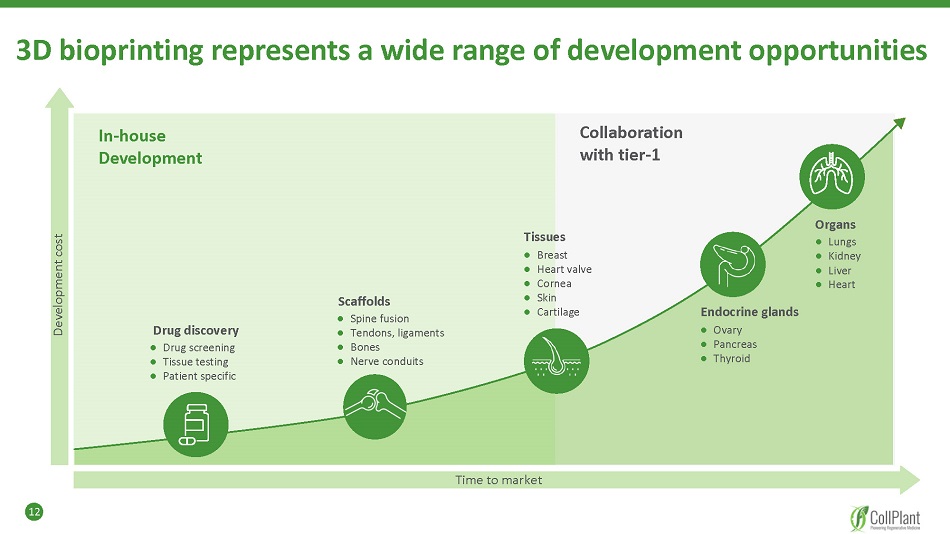
12 In - house Development Collaboration with tier - 1 Development cost Time to market Tissues ● Breast ● Heart valve ● Cornea ● Skin ● Cartilage Endocrine glands ● Ovary ● Pancreas ● Thyroid Organs ● Lungs ● Kidney ● Liver ● Heart Scaffolds ● Spine fusion ● Tendons, ligaments ● Bones ● Nerve conduits Drug discovery ● Drug screening ● Tissue testing ● Patient specific 3D bioprinting represents a wide range of development opportunities

Breast Reconstruction / Augmentation
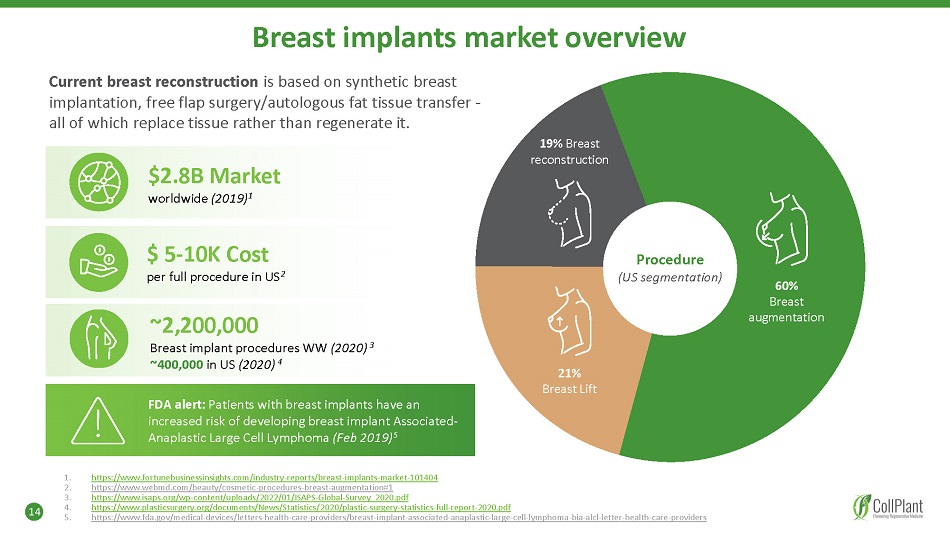
Breast implants market overview Current breast reconstruction is based on synthetic breast implantation, free flap surgery/autologous fat tissue transfer - all of which replace tissue rather than regenerate it. $2.8B Market worldwide (2019) 1 $ 5 - 10K Cost per full procedure in US 2 ~2,200,000 Breast implant procedures WW (2020) 3 ~400,000 in US (2020) 4 FDA alert: Patients with breast implants have an increased risk of developing breast implant Associated - Anaplastic Large Cell Lymphoma (Feb 2019) 5 14 1. 2. 3. 4. 5. https://www.fortunebusinessinsights.com/industry - reports/breast - implants - market - 101404 https://www.webmd.com/beauty/cosmetic - procedures - breast - augmentation#1 https://www.isaps.org/wp - content/uploads/2022/01/ISAPS - Global - Survey_2020.pdf https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic - surgery - statistics - full - report - 2020.pdf https://www.fda.gov/medical - devices/letters - health - care - providers/breast - implant - associated - anaplastic - large - cell - lymphoma - bia - alcl - letter - health - care - providers Procedure (US segmentation) 60% Breast augmentation 21% Breast Lift 19% Breast reconstruction
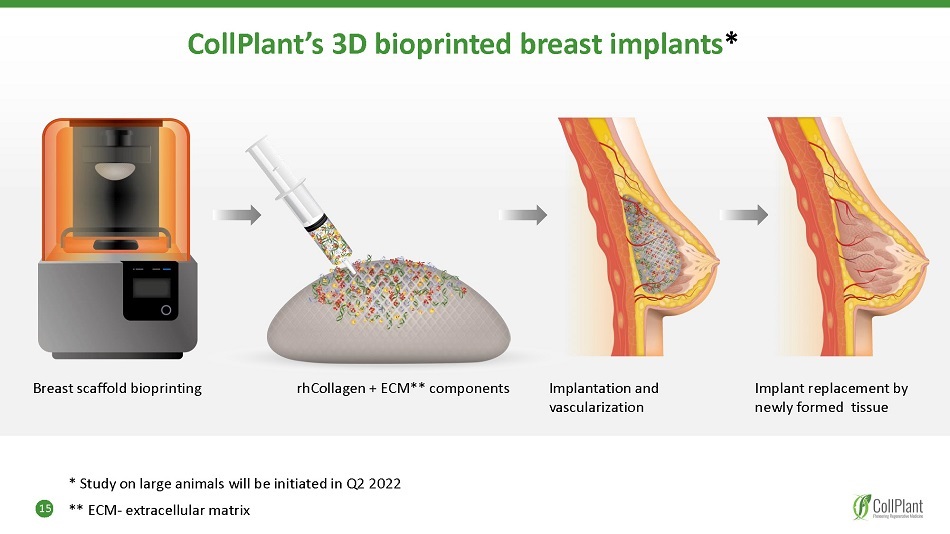
15 CollPlant’s 3D bioprinted breast implants * Breast scaffold bioprinting rhCollagen + ECM** components Implantation and vascularization Implant replacement by newly formed tissue * Study on large animals will be initiated in Q2 2022 ** ECM - extracellular matrix

Provide extra coverage, direct - to - implant reconstruction and superior cosmetic results US: mostly human - sourced (93%) EU: mostly animal - sourced Used in 61% of U.S. breast reconstruction surgeries Breast reconstruction procedures using ADMS /yr (US): 60K Market potential (US) $600Mn Surgical matrixes derived from cadavers and animals are associated with high costs, supply shortage and batch - to - batch variability 16 rhCollagen - based regenerative tissue matrix Regenerative Tissue Matrix (RTM) Developed in collaboration with 3D Systems
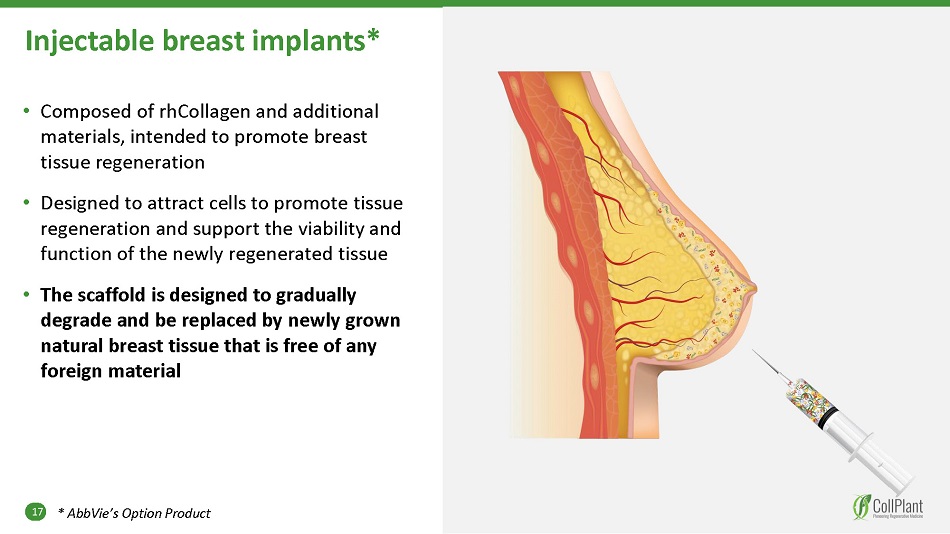
Injectable breast implants* 17 • Composed of rhCollagen and additional materials, intended to promote breast tissue regeneration • Designed to attract cells to promote tissue regeneration and support the viability and function of the newly regenerated tissue • The scaffold is designed to gradually degrade and be replaced by newly grown natural breast tissue that is free of any foreign material * AbbVie’s Option Product

rhCollagen BioInk compositions in development Direct Ink Writing (DIW) Inkjet Projection Stereolithography Laser Induced Forward Transfer (LIFT) Polymers (physical properties) Ceramics (hard tissue) Nanoparticles (sustained release/ physical properties ( ECM components (biological/ physical properties)
CollPlant now offers Collink.3D TM , human collagen bioink platform, for 3D bioprinting of organs, scaffolds and tissue models for drug discovery, personalized medicine and regenerative medicine applications. 19

Our Partners Companies Consortiums & organizations Development and commercialization agreement Joint development agreement Universal BioInk Industry committee Scalable manufacturing of tissue engineered products 20 Collaboration agreement Supply agreement

Yehiel Tal CEO Regentis Biomaterials ProChon Biotech Kulicke & Soffa Industries Eran Rotem Deputy CEO & CFO Tefron, CFO (NYSE,TASE) Healthcare Tech., CFO (NASDAQ) & Gamida E&Y Ilana Belzer, PhD COO BioHarvest Procognia Ltd. Omrix Biopharmaceuticals Interpharm Michal Roytman VP Sales & Marketing Ocon Medical Neuroderm (now Mitsubishi Tanabe) Neurim Pharmaceuticals Frutarom (now IFF) 21 Philippe Bensimon, PharmD VP RA/QA/CA Maquet Getinge 3M Medical Experienced management team Hadas Dreiher - Horowitz VP HR Elbit Teva Pharmaceuticals Mul - T - Lock

CollPlant investment thesis Only commercially viable technology currently available that can produce truly human collagen Multi - billion dollar markets: innovative rhCollagen products initially aimed at 3D bioprinting and medical aesthetics Broadly applicable technology: Ideal building block/scaffolding molecule for regenerative medicine Strategic agreement with AbbVie and 3D Systems Clinically validated technology Proven management team 22

Thank you