
Exhibit 99.1

1 APRIL 202 3 NASDAQ: CLGN Company Overview Our Mission Developing and delivering collagen technology and regenerative medicine products to improve and prolong lives

2 Certain statements in this presentation constitute "forward - looking statements" within the meaning of Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act and are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or similar expressions . We intend these forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions . These forward - looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition, expected capital needs and expenses, statements relating to the research, development, completion and use of our products, and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future . These forward - looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made . Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achie v ed . Furthermore, actual results may differ materially from those described in the forward - looking statements and are expected to be affected by a variety of risks and factors that are beyond our control . Risks and uncertainties for our company include, but are not limited to : the Company ’ s history of significant losses and its need to raise additional capital and its inability to obtain additional capital on acceptable terms, or at all ; the Company ’ s expectations regarding the timing and cost of commencing clinical trials with respect to tissues and organs which are based on its rhCollagen based Bioink and products for medical aesthetics ; the Company ’ s ability to obtain favorable pre - clinical and clinical trial results ; regulatory action with respect to rhCollagen based BioInkand medical aesthetics products including but not limited to acceptance of an application for marketing authorization, review and approval of such application, and, if approved, the scope of the approved indication and labeling ; commercial success and market acceptance of the Company ’ s rhCollagen based products, in 3 D bioprinting and medical aesthetics ; the Company ’ s ability to establish sales and marketing capabilities or enter into agreements with third parties and its reliance on third party distributors and resellers ; the Company ’ s ability to establish and maintain strategic partnerships and other corporate collaborations ; the Company ’ s reliance on third parties to conduct some or all aspects of its product manufacturing ; the scope of protection the Company is able to establish and maintain for intellectual property rights and the Company ’ s ability to operate its business without infringing the intellectual property rights of others ; the overall global economic environment ; the impact of competition and new technologies ; general market, political, and economic conditions in the countries in which the Company operates ; projected . capital expenditures and liquidity ; changes in the Company ’ s strategy ; and litigation and regulatory proceedings . Many of these factors that will determine actual results are beyond our ability to control or predict . For a discussion of the factors that may cause our actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied in such forward - looking statements, see the “ Risk Factors ” section of included in our most recently filed Annual Report on Form 20 - F . Existing and prospective investors are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof . The statements made in this presentation speak only as of the date stated herein, and subsequent events and developments may cause our expectations and beliefs to change . Unless otherwise required by applicable securities laws, we do not intend, nor do we undertake any obligation, to update or revise any forward - looking statements contained in this presentation to reflect subsequent information, events, results or circumstances or otherwise . While we may elect to update these forward - looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise, except as required by law . The trademarks included herein are the property of the owners thereof and are used for reference purposes only . Such use should not be construed as an endorsement of such products . Safe Harbor Statement

3 Imagine a future where… There will be an unlimited supply of spare parts for the human body , including life - saving organs Medical treatment will be tailored for the individual characteristics of each patient Drugs will be developed without the need for animal testing We aspire to become the leaders in regenerative medicine, helping people live longer and better and creating improvements in science through our regenerative technology Our Company Vision
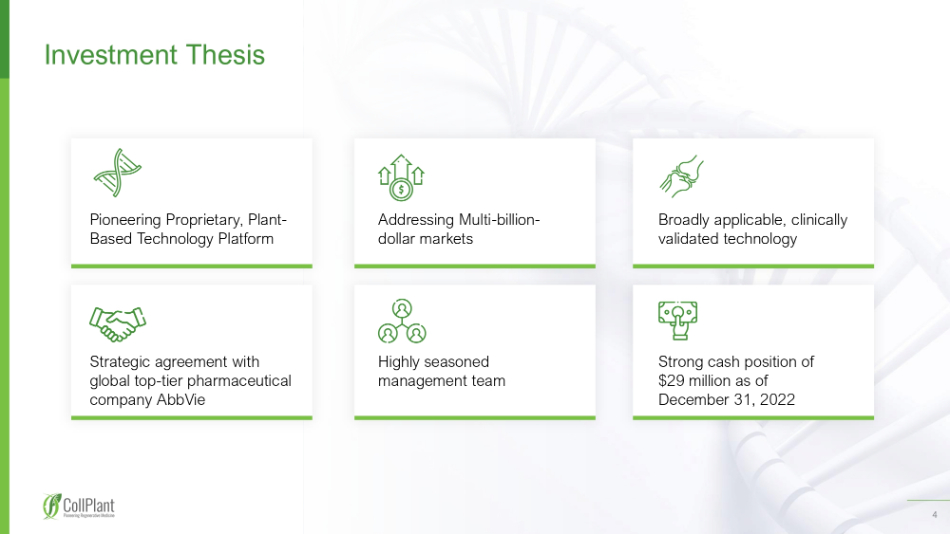
4 I nvestment T hesis Pioneering Proprietary, Plant - Based Technology Platform Addressing Multi - billion - dollar markets Broadly applicable, clinically validated technology Strategic agreement with global top - tier pharmaceutical company AbbVie Highly seasoned management team Strong cash position of $29 million as of December 31, 2022

5 At - a Glance • * Figures as of April 4 , 2023 NASDAQ (CLGN) (listed since 2018) cGMP production facility that utilizes proprietary production processes Shares outstanding: ~ 11 M Well - capitalized Market cap*: ~$ 80 M* Avg trading vol ( 3 m)*: 15 M shares/day Clinically validated in Europe Re - orient; fully vertical operation Headquarters Rehovot, Israel ~ 70 Employees
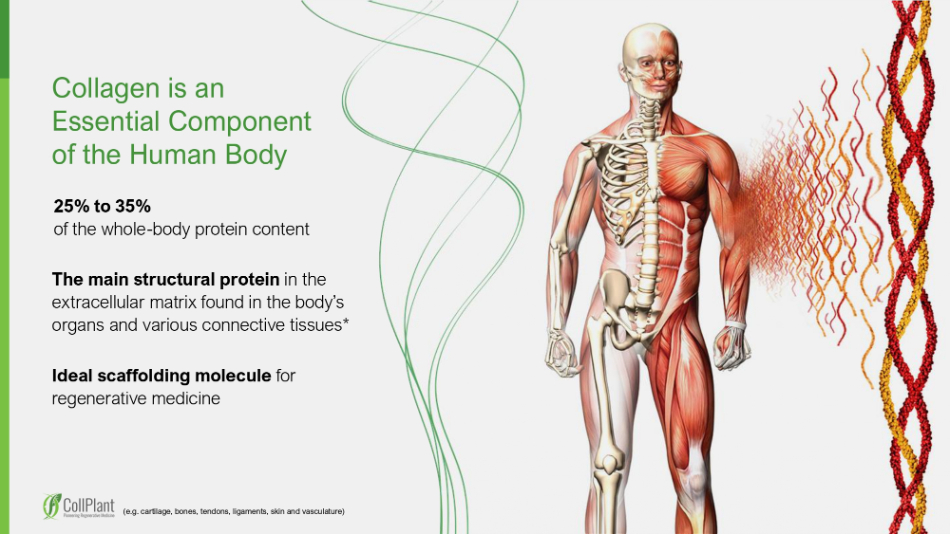
6 Collagen is an Essential Component of the Human Body (e.g. cartilage, bones, tendons, ligaments, skin and vasculature) 25 % to 35 % of the whole - body protein content Ideal scaffolding molecule for regenerative medicine The main structural protein in the extracellular matrix found in the body ’ s organs and various connective tissues* ADvD0

7 4 Five human genes essential to the synthesis of Type 1 collagen are introduced into tobacco plants to produce rhCollagen identical to human collagen but without an adverse immune response Our Technology Platform Produces Human Collagen in Plants at Mass - Scale N - propeptide C - propeptide COL1 ⍺ 1 COL1 ⍺ 2 P 4 H ⍺ P 4 Hß LH 3
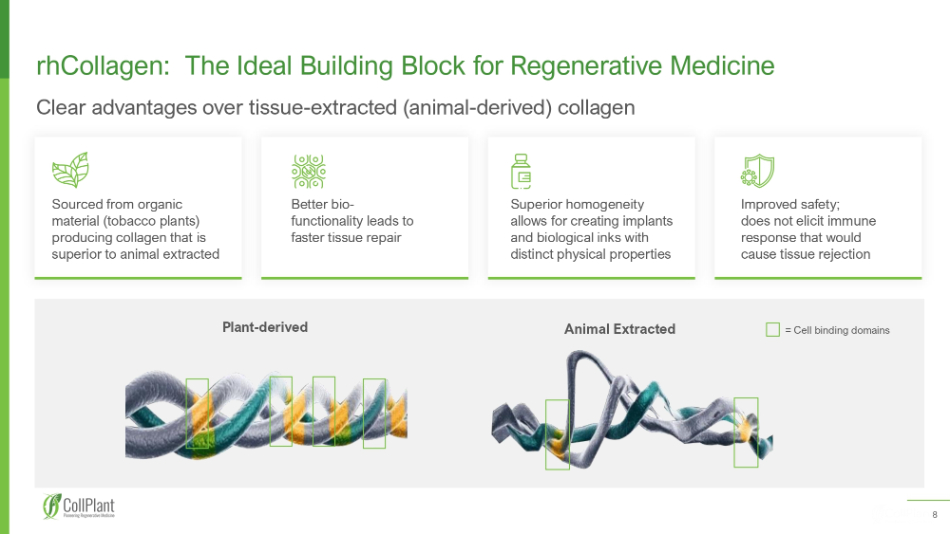
8 5 Animal Extracted Plant - derived = Cell binding domains rhCollagen: The I deal B uilding B lock for Regenerative Medicine Clear advantages over tissue - extracted (animal - derived) collagen Superior homogeneity allows for creating implants and biological inks with distinct physical properties Improved safety; does not elicit immune response that would cause tissue rejection Sourced from organic material (tobacco plants) producing collagen that is superior to animal extracted Better bio - functionality leads to faster tissue repair
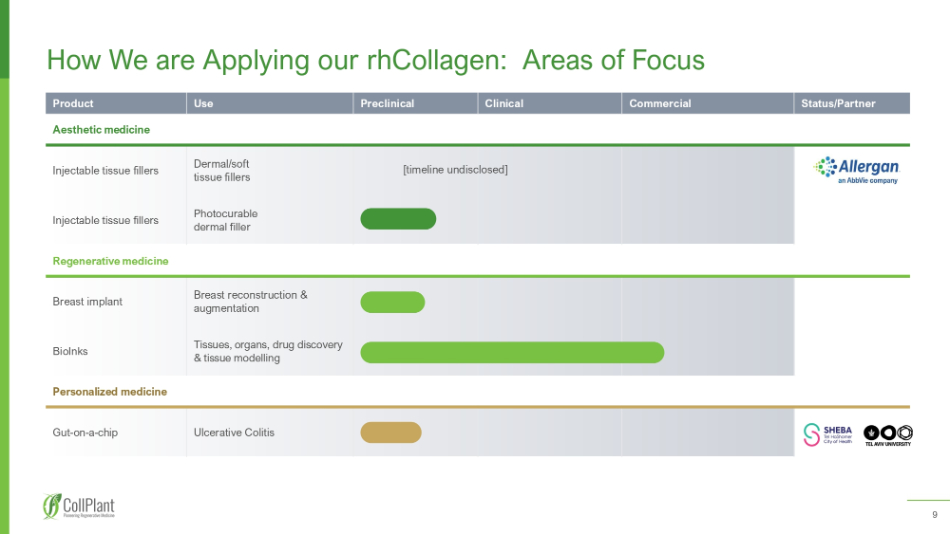
9 Product Use Preclinical Clinical Commercial Status/Partner Aesthetic medicine Injectable tissue fillers Dermal/soft tissue fillers Injectable tissue fillers Photocurable dermal filler Regenerative medicine Breast implant Breast reconstruction & augmentation BioInks Tissues, organs, drug discovery & tissue modelling Personalized medicine Gut - on - a - chip Ulcerative Colitis How We are Applying our rhCollagen : Areas of Focus [timeline undisclosed]
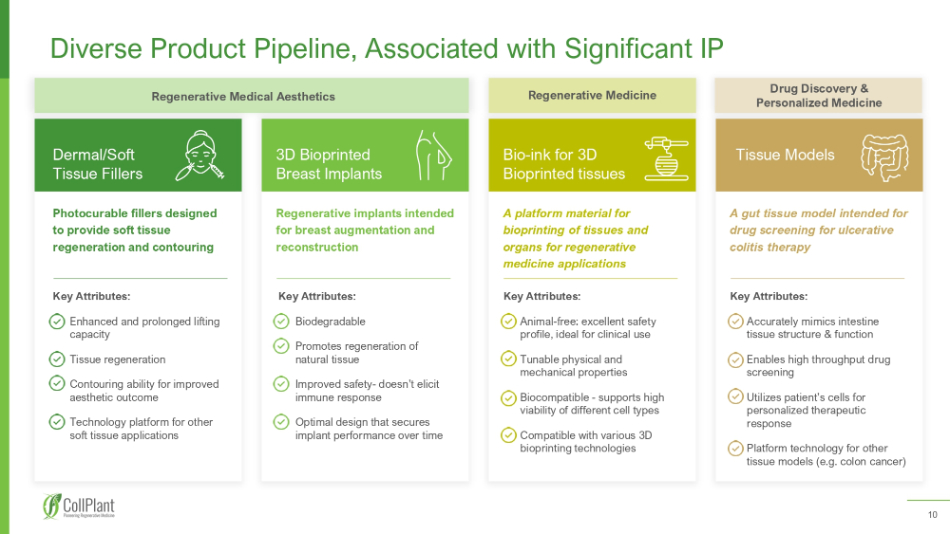
10 Diverse Product Pipeline, Associated with Significant IP Photocurable fillers designed to provide soft tissue regeneration and contouring Dermal/Soft Tissue Fillers Key Attributes: Enhanced and prolonged lifting capacity Tissue regeneration Contouring ability for improved aesthetic outcome Technology platform for other soft tissue applications Regenerative implants intended for breast augmentation and reconstruction 3D Bioprinted Breast Implants Key Attributes: Biodegradable Promotes regeneration of natural tissue Improved safety - doesn’t elicit immune response Optimal design that secures implant performance over time A platform material for bioprinting of tissues and organs for regenerative medicine applications Bio - ink for 3D Bioprinted tissues A gut tissue model intended for drug screening for ulcerative colitis therapy Tissue Models Key Attributes: Animal - free: excellent safety profile, ideal for clinical use Tunable physical and mechanical properties Biocompatible - supports high viability of different cell types Compatible with various 3D bioprinting technologies Key Attributes: Accurately mimics intestine tissue structure & function Enables high throughput drug screening Utilizes patient’s cells for personalized therapeutic response Platform technology for other tissue models (e.g. colon cancer) Regenerative Medical Aesthetics Regenerative Medicine Drug Discovery & Personalized Medicine
11 Dermal/Soft Tissue Fillers In collaboration with
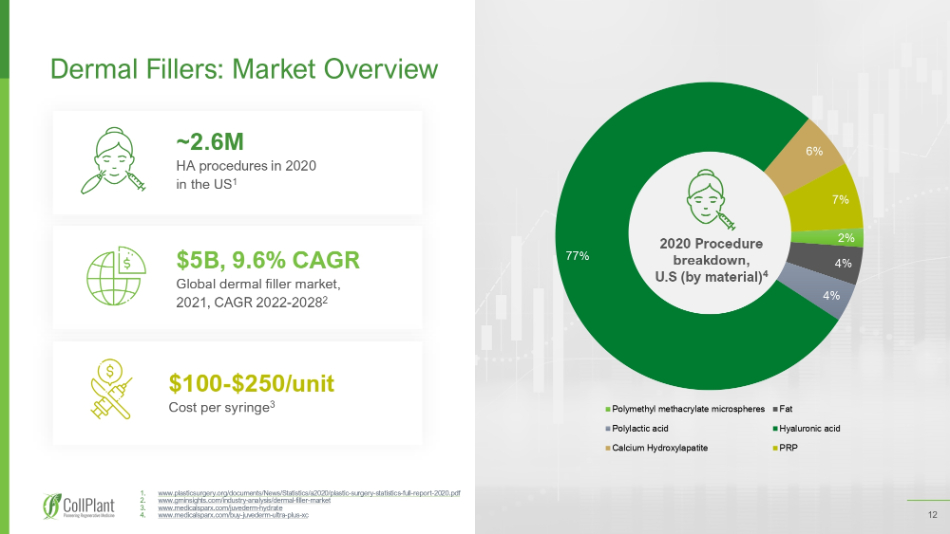
12 12 1. www.plasticsurgery.org/documents/News/Statistics/a2020/plastic - surgery - statistics - full - report - 2020.pdf 2. www.gminsights.com/industry - analysis/dermal - filler - market 3. www.medicalsparx.com/juvederm - hydrate 4. www.medicalsparx.com/buy - juvederm - ultra - plus - xc Dermal Fillers: Market Overview ~2.6 M HA procedures in 2020 in the US 1 $5B, 9.6% CAGR Global dermal filler market, 2021, CAGR 2022 - 2028 2 $ 100 - $ 250 /unit Cost per syringe 3 2% 4% 4% 77% 6% 7% Polymethyl methacrylate microspheres Fat Polylactic acid Hyaluronic acid Calcium Hydroxylapatite PRP 2020 Procedure breakdown, U.S (by material) 4
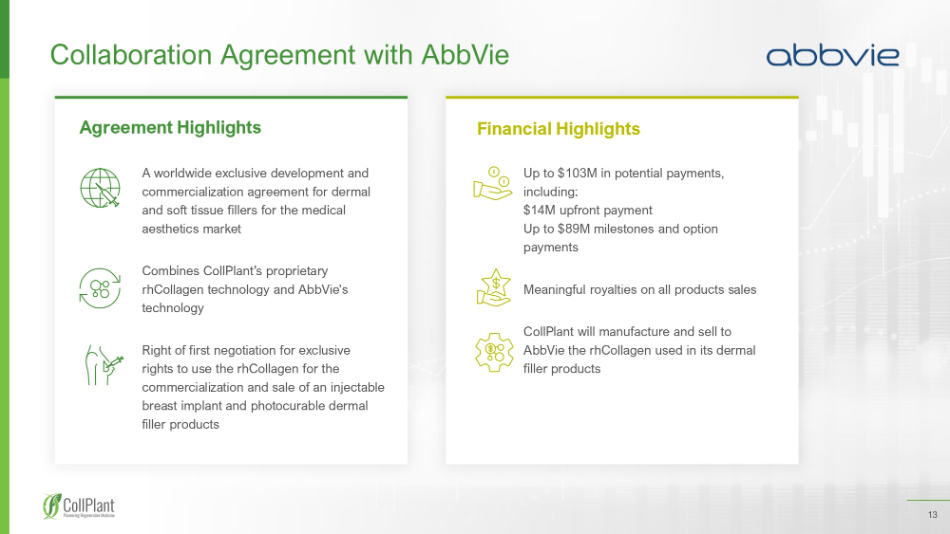
13 Collaboration Agreement with AbbVie Agreement Highlights Financial Highlights A worldwide exclusive development and commercialization agreement for dermal and soft tissue fillers for the medical aesthetics market Combines CollPlant ’ s proprietary rhCollagen technology and AbbVie's technology Right of first negotiation for exclusive rights to use the rhCollagen for the commercialization and sale of an injectable breast implant and photocurable dermal filler products Up to $103M in potential payments, including: $14M upfront payment Up to $89M milestones and option payments Meaningful royalties on all products sales CollPlant will manufacture and sell to AbbVie the rhCollagen used in its dermal filler products

14 14 Unmet Need: Dermal Fillers To - Date Have Numerous Drawbacks Safety issues • Various adverse events, including inflammatory response • Potential for nodule formation Undesired physical outcome (unnatural look due to lack of pliability under skin) Short - lasting and require repeat injections

15 The New Shape of Beauty Photocurable Dermal & Soft Tissue Filler
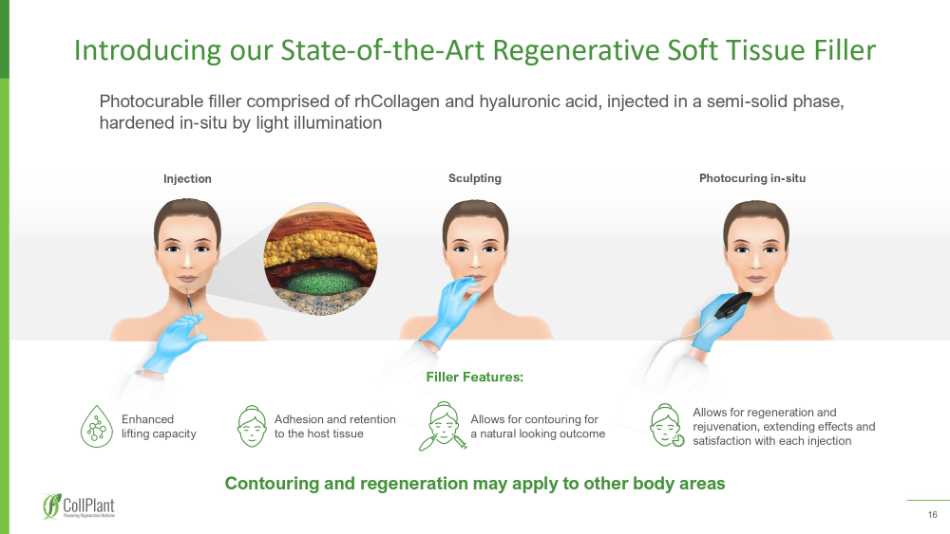
16 Injection Sculpting Photocuring in - situ Filler Features: Contouring and regeneration may apply to other body areas Allows for regeneration and rejuvenation, extending effects and satisfaction with each injection Enhanced lifting capacity Adhesion and retention to the host tissue Allows for contouring for a natural looking outcome Introducing our State - of - the - Art Regenerative Soft Tissue Filler Photocurable filler comprised of rhCollagen and hyaluronic acid, injected in a semi - solid phase, hardened in - situ by light illumination
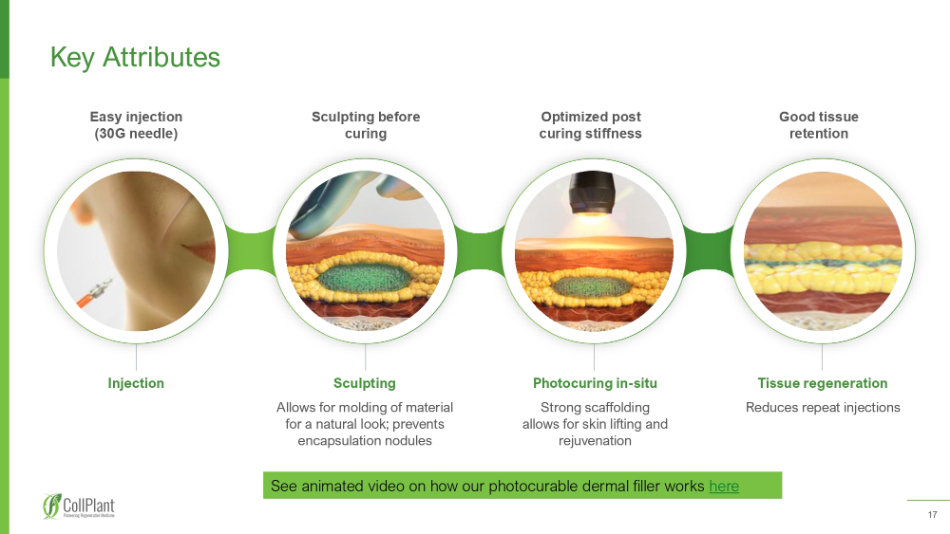
17 Key Attributes Injection Sculpting Allows for molding of material for a natural look; prevents encapsulation nodules Photocuring in - situ Strong scaffolding allows for skin lifting and rejuvenation Tissue regeneration Reduces repeat injections Easy injection (30G needle) Sculpting before curing Optimized post curing stiffness Good tissue retention See animated video on how our photocurable dermal filler works here

18 Currently HA marketed products CONTOURA ® Lifting Rejuvenation Contouring Evolution of Hyaluronic Acid (HA) Dermal Filler

19 Breast Reconstruction / Augmentation CollPlant’s first - ever regenerating breast implant
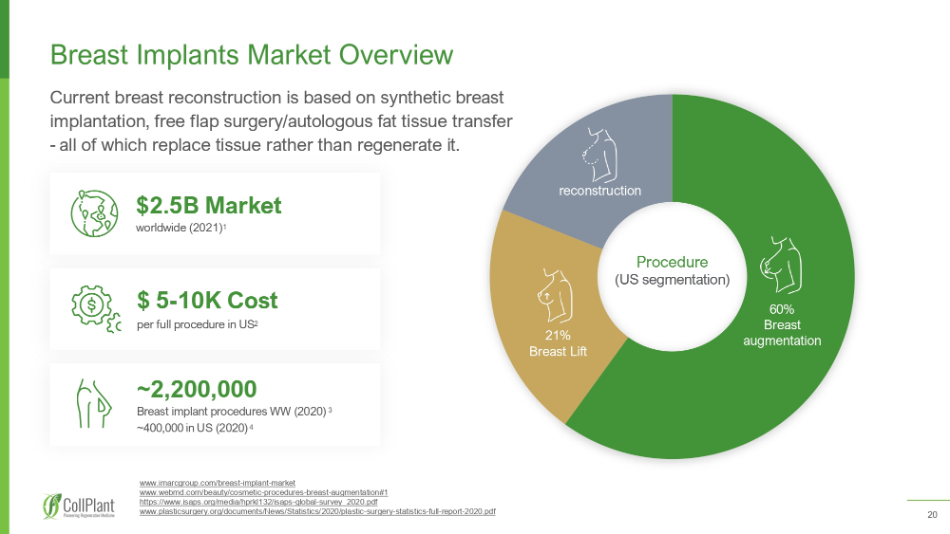
20 Breast Implants Market Overview 60 % Breast augmentation 21 % Breast Lift reconstruction Procedure (US segmentation) Current breast reconstruction is based on synthetic breast implantation, free flap surgery/autologous fat tissue transfer - all of which replace tissue rather than regenerate it. ~2,200,000 Breast implant procedures WW (2020) 3 ~400,000 in US (2020) 4 $2.5B Market worldwide (2021) 1 $ 5 - 10K Cost per full procedure in US 2 www.imarcgroup.com/breast - implant - market www.webmd.com/beauty/cosmetic - procedures - breast - augmentation# 1 https://www.isaps.org/media/hprkl 132 /isaps - global - survey_ 2020 .pdf www.plasticsurgery.org/documents/News/Statistics/ 2020 /plastic - surgery - statistics - full - report - 2020 .pdf

21 Unmet Need: The Ability to Regenerate Breast Tissue No regenerative breast implant exists FDA alert: Patients with breast implants have an increased risk of developing breast implant Associated - Anaplastic Large Cell Lymphoma (Feb 2019) * * https://www.fda.gov/medical - devices/breast - implants/medical - device - reports - breast - implant - associated - anaplastic - large - cell - lymphoma
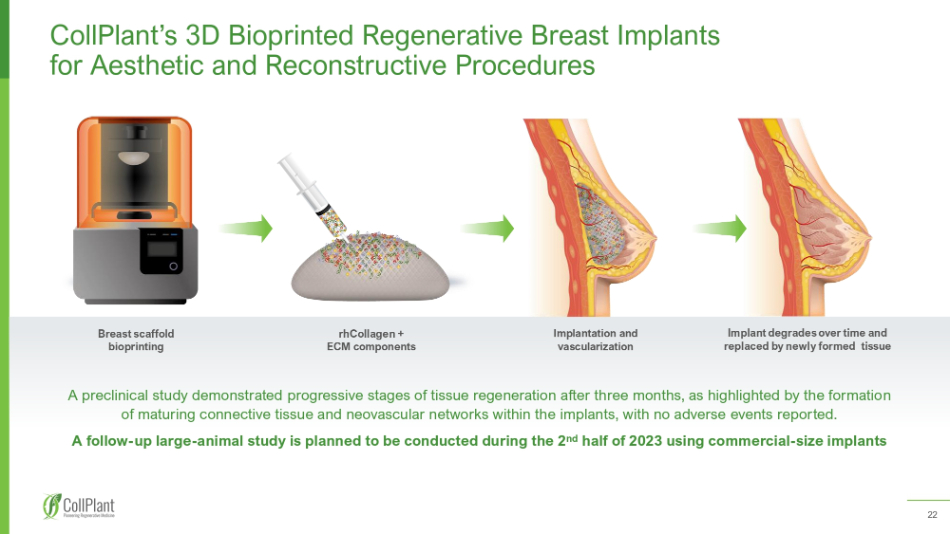
22 Breast scaffold bioprinting rhCollagen + ECM components Implantation and vascularization Implant degrades over time and replaced by newly formed tissue CollPlant ’ s 3 D Bioprinted Regenerative Breast Implants for Aesthetic and Reconstructive Procedures A preclinical study demonstrated progressive stages of tissue regeneration after three months, as highlighted by the formatio n of maturing connective tissue and neovascular networks within the implants, with no adverse events reported. A follow - up large - animal study is planned to be conducted during the 2 nd half of 2023 using commercial - size implants

23 Joint Development and Commercialization Agreement with Stratasys: Announced 4/4/2023 Co - development agreement with a leader in additive manufacturing with decades of 3 D printing experience Under the agreement, both companies have agreed to cross - promote each other’s bioprinting products Combines the technologies of Stratasys ’ new bioprinter based on its precise P 3 w 3 D printing technology with CollPlant ’ s rhCollagen - based bioinks Agreement terms CollPlant and Stratasys have a joint development and commercialization agreement to collaborate on the development of a solution to bio - fabricate human tissues and organs The first project focuses on the development of an industrial - scale solution to produce CollPlant’s regenerative, first - ever breast implant based on its rhCollagen technology.

24 Gut - on - a - C hip

25 Our rhCollagen - B ased 3 - D B ioprinted G ut - on - a - C hip Has the Potential to Shift D rug D iscovery and P ersonalized M edicine https://www.congress.gov/bill/ 117 th - congress/house - bill/ 2565 /text?r= 76 &s= 1 https://www.science.org/content/article/fda - no - longer - needs - require - animal - tests - human - drug - trials Animal models are wrong more often than right…” Don E. Ingber, M.D., Ph.D., The Wyss Institute for Biologically Inspired Engineering at Harvard University Chip technologies offer significant potential to change the diagnostic paradigm and personalized treatment landscape with both refined and cost - effective laboratory testing H.R. 2565 - FDA Modernization Act of 2021 passed in January 2023 , amends the Federal Food, Drug, and Cosmetic Act to allow manufacturers and sponsors of a drug to use alternative testing methods to animal testing to investigate the safety and effectiveness of a drug, and for other purposes . “

26 Inflammatory Bowl Diseases: An Example of an Unmet Need that Exists for IBD Patients Inflammatory bowel diseases, which include ulcerative colitis and Crohn’s disease, are characterized by chronic inflammation, a relapsing and remitting clinical course and life - long treatment. > 6 M ulcerative colitis patients worldwide No cure Individualized In treating each patient, some fail to respond Limited models In predicting therapeutic response, this results in exposure to unjustified drugs and a delay in treatment There is a need for novel personalized platforms to improve therapeutic choices and patient outcome

27 Our Collaborators: Gut - on - a - Chip Technology From Tel - Aviv University and Sheba Medical Center Co - development agreement with Tel - Aviv University and Sheba Medical Center Model designed to accurately mimic the human intestine tissue structure and function Patient - specific cells enable screening of multiple drugs and identification of the most effective personalized therapeutic response Agreement terms (Nov 2022 ) CollPlant has an exclusive license for development, manufacturing and commercializing of the final product; Tel Aviv University and Sheba will receive royalties on product sales CollPlant is open to partnering this program for commercialization 27

28 3 - D Bioprinted Tissues and Organs
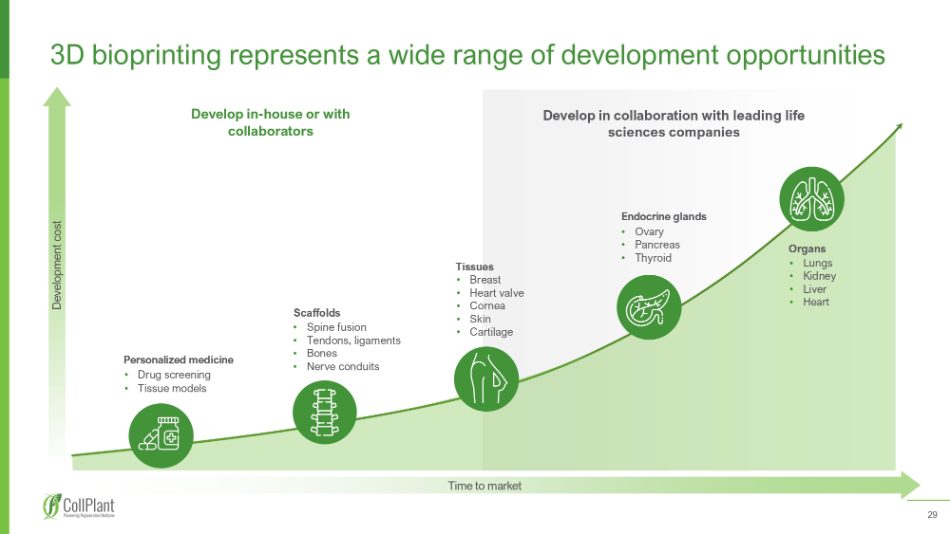
29 3 D bioprinting represents a wide range of development opportunities Develop in - house or with collaborators Develop in c ollaboration with leading life sciences companies Development cost Time to market Tissues • Breast • Heart valve • Cornea • Skin • Cartilage Endocrine glands • Ovary • Pancreas • Thyroid Organs • Lungs • Kidney • Liver • Heart Scaffolds • Spine fusion • Tendons, ligaments • Bones • Nerve conduits Personalized medicine • Drug screening • Tissue models

30 rhCollagen - based BioInks enable high resolution printing of elastic scaffolds

31 Bioinks Competitive Landscape Collagen - based: • Tissue - extracted collagen (e.g. rat, bovine) • Synthetic peptides Drawbacks of most commonly used bioinks: • Unsuitable for clinical use • May elicit immune response • High batch - to - batch variability • Small scale production Non - collagen - based: • Polysaccharides (HA, cellulose, alginate) • Glycoprotein (Fibrinogen) • Synthetic peptides • Synthetic polymers (PEG, PCL, Pluronic)

32 Collink. 3 D : rhCollagen - bioink platform for biofabrication CollPlant Collink. 3 D TM : A xeno - free human - collagen based BioInk, perfectly mimicking properties of the native tissue or organ Cytocompatible , Biofunctional Animal - free : excellent safety profile non immunogenic Optimal rheology at room temperature Compatible with major printing technologies Mass production - consistency robustness High homogeneity reproducibility

33 rhCollagen BioInk components Direct Ink Writing (DIW) Inkjet Projection Stereolithography Laser Induced Forward Transfer (LIFT) Polymers (physical properties) Ceramics (hard tissue) Nanoparticles (sustained release/physical properties ECM components (biological/ physical properties) CollInk - 3D 20ml

34 Seasoned Management Team with Engineering, Pharmaceutical, Device and Life Sciences Experience Yehiel Tal CEO Regentis Biomaterials ProChon Biotech Kulicke & Soffa Industries Eran Rotem Deputy CEO & CFO Tefron , CFO (NYSE,TASE) Healthcare Tech., CFO (NASDAQ) & Gamida E&Y Elana Gazal , PhD VP R&D Neuroderm (now Mitsubishi Tanabe) Waters IS Foamix (now Wyne ) Beckman Coulter Michal Roytman VP Sales & Marketing Ocon Medical Neuroderm (now Mitsubishi Tanabe) Neurim Pharmaceuticals Frutarom (now IFF) Oren Fahimipoor VP Operations Omrix Biopharmaceuticals (J&J) Teva Pharmaceutical Industries Ltd Philippe Bensimon, PharmD VP RA/QA/CA Maquet Getinge 3 M Medical Hadas Dreiher - Horowitz VP HR Elbit Teva Pharmaceuticals Mul - T - Lock
35 Our Partners Developme nt and commercialization agreement Joint development agreement Universal BioInk Industry committee Scalable manufacturing of tissue engineered products 23 Supply agreement Developme nt and commercialization agreement

36 I nvestment Summary Pioneering proprietary, plant - based technology platform The only commercially viable technology that can produce truly human collagen at mass scale and without reliance on animal tissue Addressing Multi - billion - dollar markets Innovative rhCollagen technology initially focused on medical aesthetic applications; differentiated, transformative, next - generation soft tissue filler is regenerative Broadly applicable, clinically validated technology Ideal building block/scaffolding molecule for regenerative medicine that has clear benefits over tissue - extracted collagen and the potential to create first - in - class products, extend product life cycles and expand applications in other areas of medicine Strategic agreement with global top - tier pharmaceutical company AbbVie Allows for up to $ 89 M in milestone payments plus additional royalties on sales Highly seasoned management team With experience in bioengineering, biomaterials, broad life sciences, pharmaceuticals and devices Strong cash position at $ 29 M as of December 31 , 2022 Zero debt as of December 2022; Collaboration agreements with well - capitalized industry leaders

37 Thank you